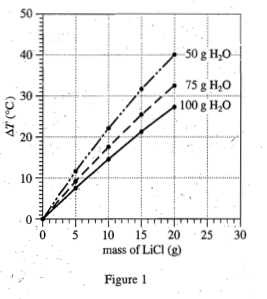
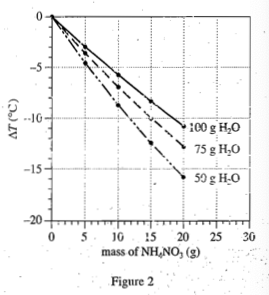
5. 5.Based on Figures 1 and 2, which of the following combinations of a solute and H2O at a known Ti would produce the greatest increase in temperature?
Your Answer is
Correct Answer is A
Explanation
Because the increase temperature is required in the question stem, you can only choose LiCl, and exclude the C&D option;
Observe figure1 again, compare the slopes of the three lines in the figure, and find that H2< The smaller the amount of /sub>0, the larger the ΔT, so choose 5g H2O
of option A