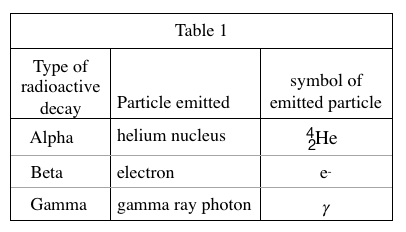
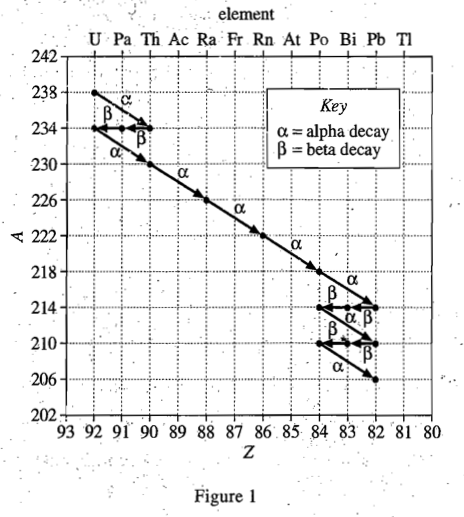
21. A sample of spent nuclear reactor fuel contains a mixture of a uranium isotope, and a plutonium isotope,
. Based on Table 1 and Figure 1, if one of the isotopes is produced by the radioactive decay of the other isotope, which of the following best explains how the mixture was formed?
Your Answer is
Correct Answer is B
Explanation
It can be seen from figure 1 that α decay will generally reduce the proton number Z of the element by 2 and the mass number A by 4. So 23994Pu after α decay will just become 23592U