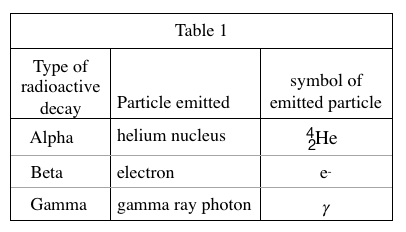
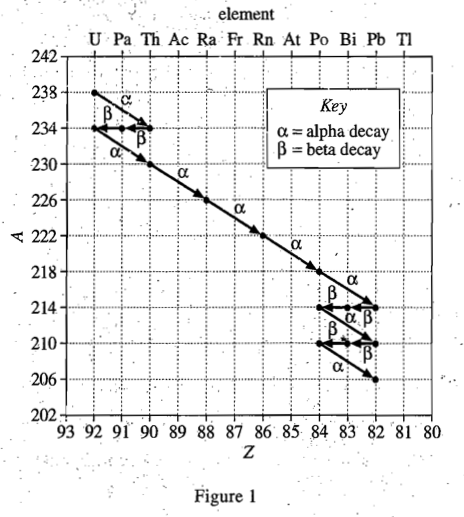
20. Based on Figure 1, if a nucleus of underwent beta decay, which of the following nuclei would be produced?
Your Answer is
Correct Answer is G
Explanation
It can be seen from table 1 that after beta decay occurs, the proton number Z increases by 1, while the mass number A remains unchanged. So the proton number (lower left corner) of the element appearing in the question stem becomes 91, and the mass number (upper right corner) is still 230