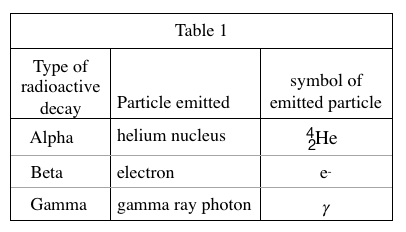
19. How many neutrons, if any, does a nucleus of the isotope of helium listed in Table 1 contain?
Your Answer is
Correct Answer is C
Explanation
In Table 1, 42The 4 in the upper left corner of He represents the proton and neutron The sum of the numbers, and the 2 in the lower right corner represents the number of protons, so the number of neutrons is equal to 4-2=2