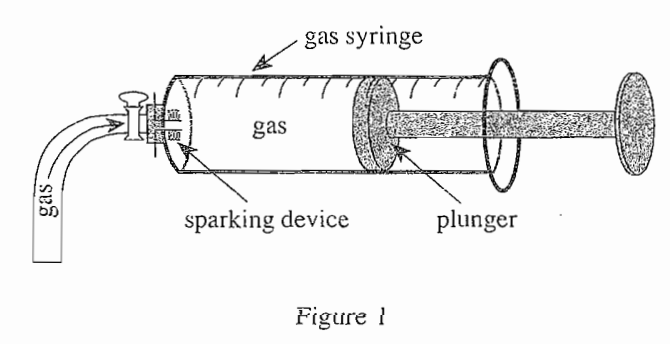
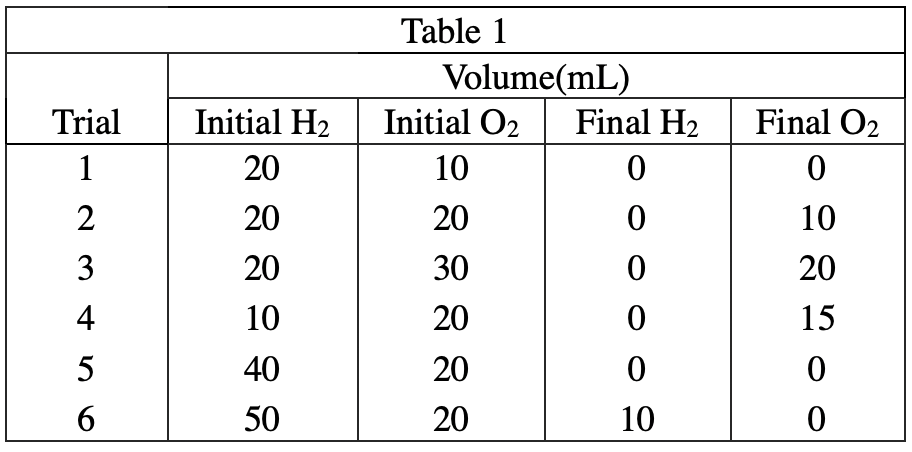
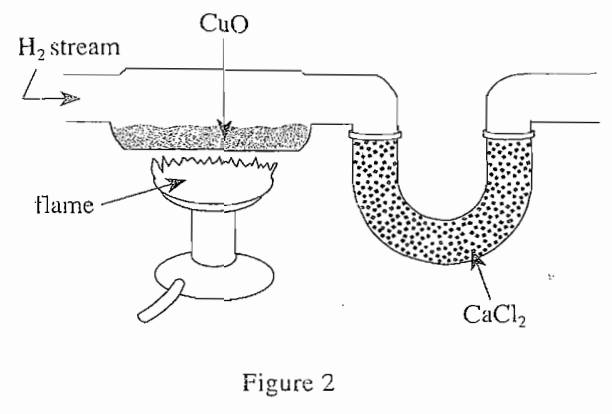
34. When nitrogen gas (N2) is reacted with H2 under certain conditions, the following reaction occurs:
N2 + 3 H2 → 2 NH3
Based on the results of Experiment 1, if 10 mL of N2 were completely reacted with 40 mL of H2 at the same pressure and temperature, what volume of H2 would remain unreacted ?Your Answer is
Correct Answer is G
Explanation
According to the reaction equation given in the question stem, the volume consumption ratio of N2 to H2 is 1:3, so after the reaction is complete, 10 ml of N 2 will react with 30 ml of H2, and the remaining 40-30=10 ml of H2 will not react