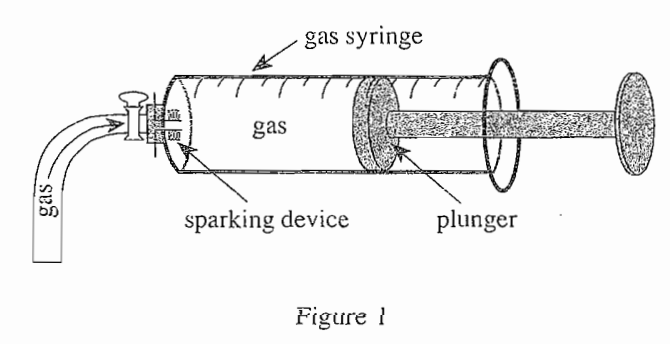
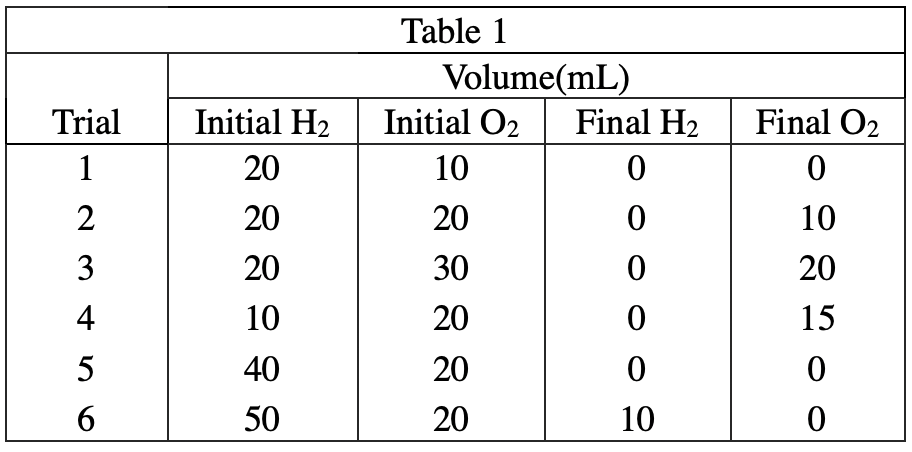
32. If 50 mL of H2 and 50 mL of O2 were reacted using the procedure from Experiment 1, the final volume of O2 would most likely be:
Your Answer is
Correct Answer is J
Explanation
It can be seen from table 1 that the consumption ratio of H2 and O2 is 2:1, so if both gases are 50mL, 25mL of O will be left after the reaction 2