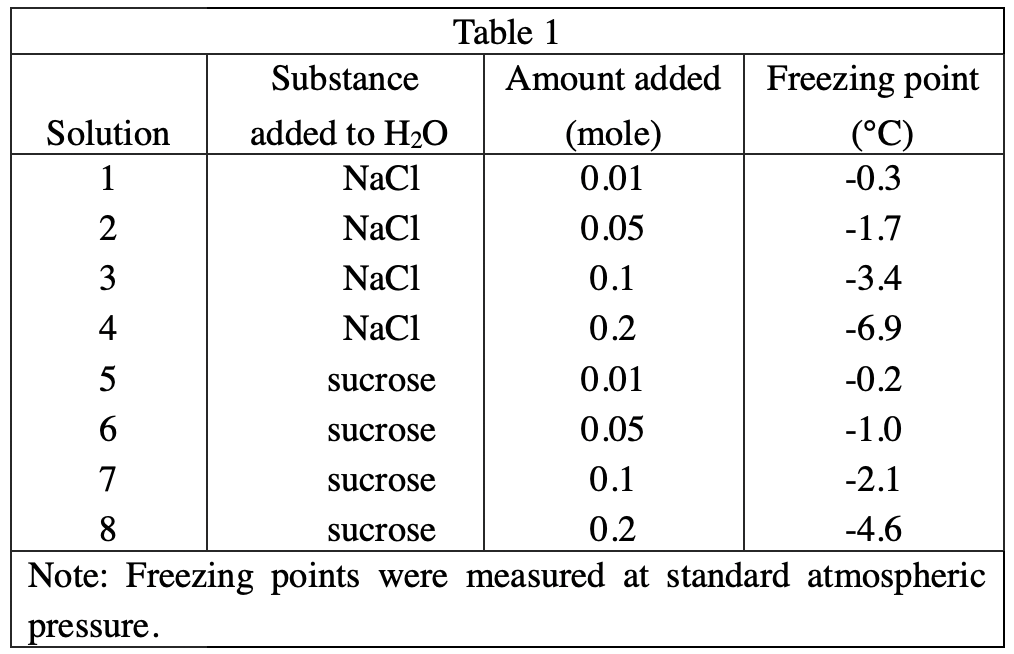
16. CaCl2 produces 3 moles of solute particles per mole when dissolved. Experiment 1 was repeated using a solution containing 100 g of H2O and 0.1 mole CaCl2. Assuming that CaCl2 has the same effect on the freezing point of H2O as does NaCl per particle produced when dissolved, the freezing point of the solution would most likely be:
Your Answer is
Correct Answer is H
Explanation
Because the question stem assumes that each particle has the same effect on the freezing point, and CaCl2 has three particles, NaCl has two, then 0.1 mole CaCl2 The effect is equivalent to that of 0.15 mole NaCl. So the freezing point should be between -3.4 and -6.9°C