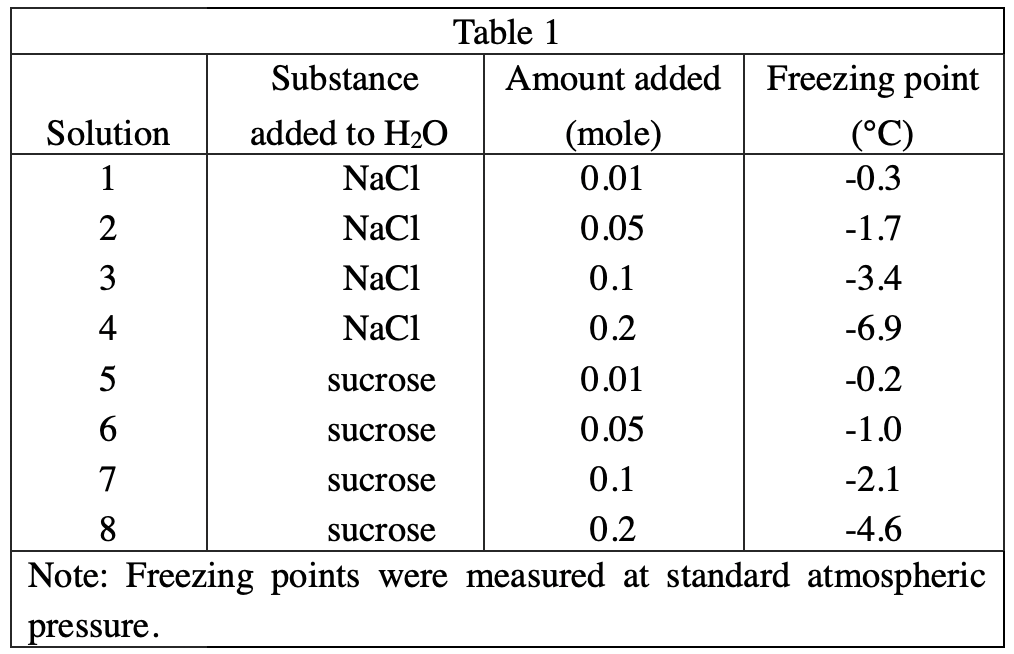
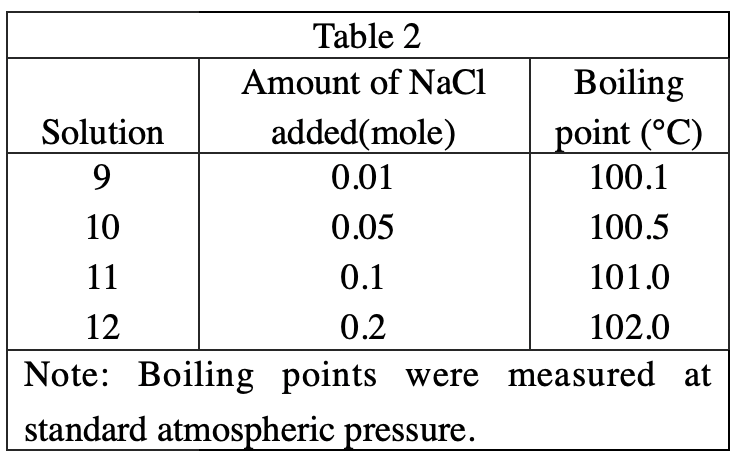
14. According to the results of Experiments 1 and 2, which of the following conclusions can be made about the magnitudes of the changes in the boiling point and freezing point of H2O solutions when 0.2 mole of NaCl is added to 100 g of H2O ? The freezing point is:
Your Answer is
Correct Answer is J
Explanation
Observing table 1 & table 2, it can be found that after adding 0.2 mole of NaCl, the freezing point decreased by 6.9°C, while the boiling point increased by 2.0°C. So the freezing point drops more than the boiling point rises