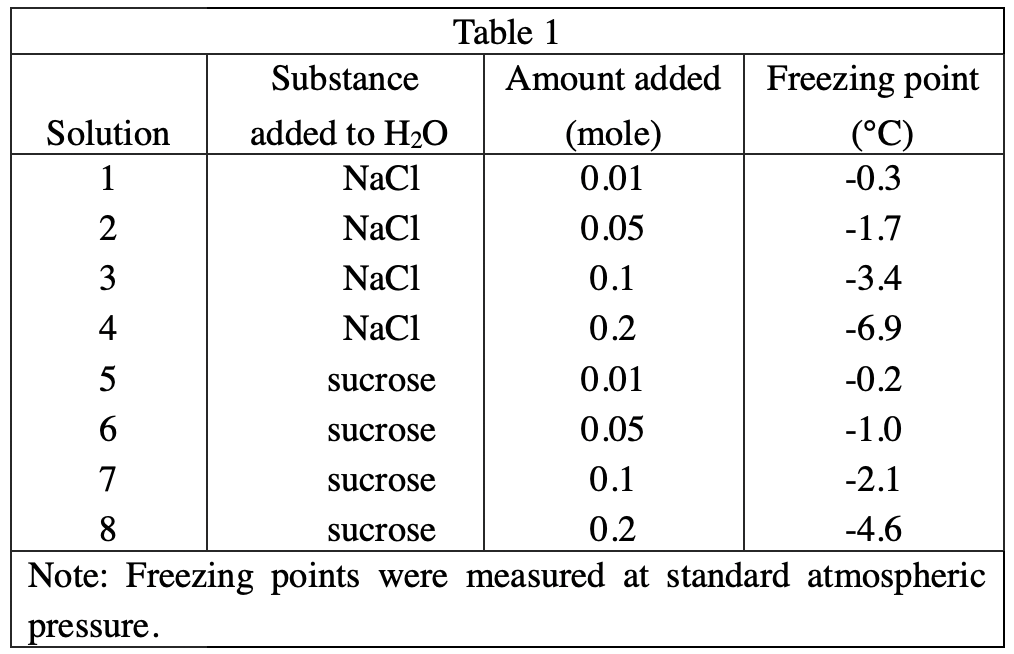
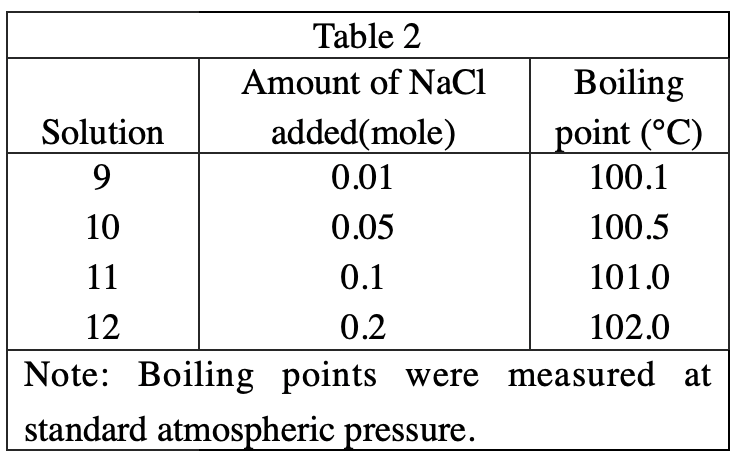
11. A solution containing 100 g of H2O and an unknown amount of NaCl boils at 104°C. Based on the results of Experiment 2, the number of moles of NaCl dissolved in the solution is closest to:
Your Answer is
Correct Answer is C
Explanation
From the data in table 2, it can be inferred that for every 0.01 mole of NaCl added, the boiling point will increase by 0.1. So when the boiling point is 104°C, 0.4 mole of NaCl should be added