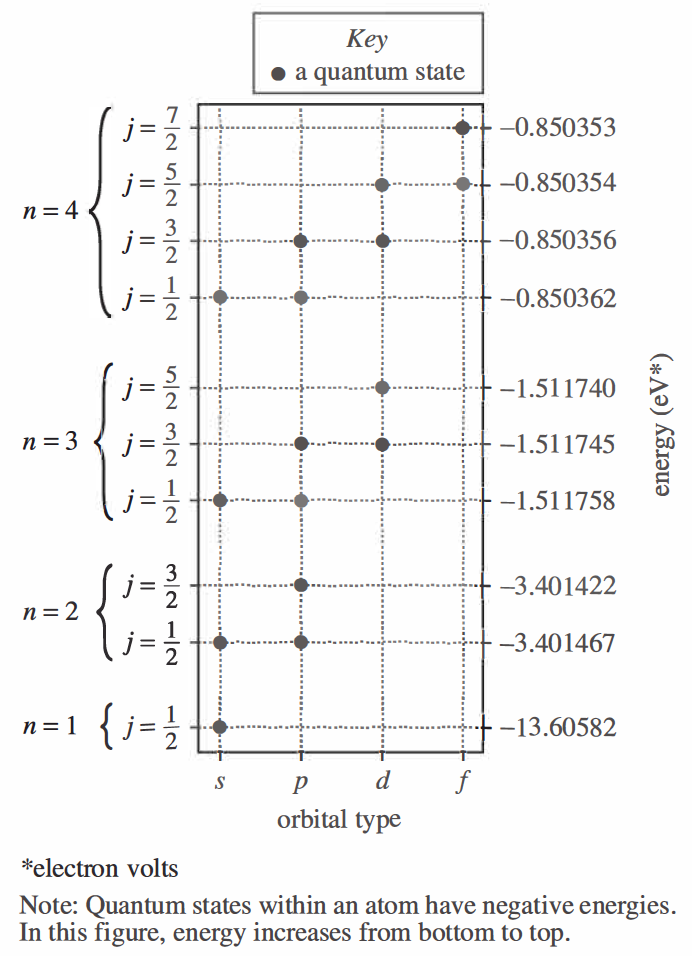
10. The first 5 orbital types are s, p, d, f, and g, in order of increasing complexity. Based on the figure, the quantum states associated with g-type orbitals would most likely have energies that are:
Your Answer is
Correct Answer is H
Explanation
As the orbit type moves from left to right, it can be seen that the corresponding energy gradually increases towards 0, so it should be greater than -0.85 at g; and because the first sentence of the background text field indicates the energy of electrons, electrons can only Negative charge is therefore less than 0, choose H