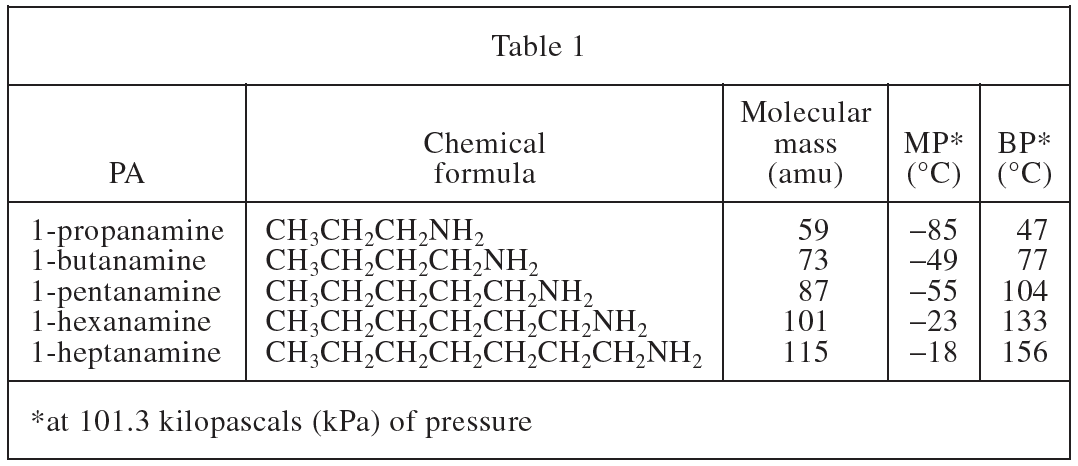
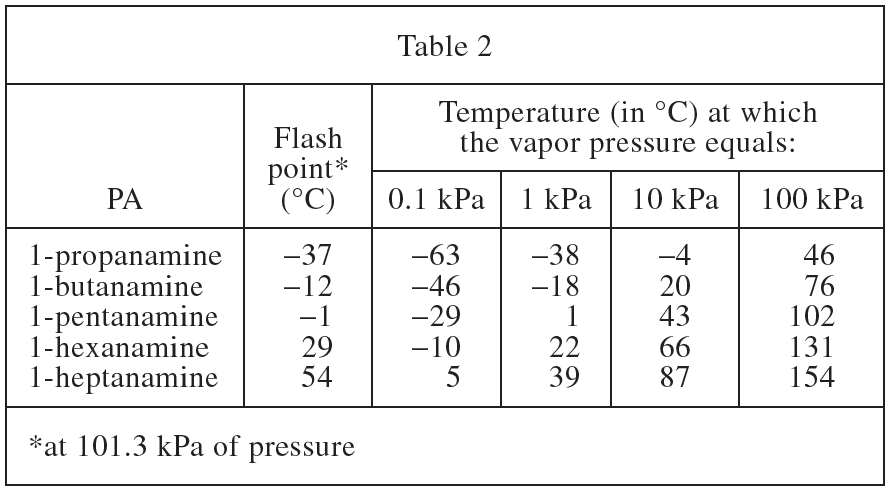
11. Consider the PA 1-octanamine, CH3(CH2)7NH2. Based on Tables 1 and 2, the temperature at which the vapor pressure of 1-octanamine equals 100 kPa would most likely be:
Your Answer is
Correct Answer is D
Explanation
PA 1-octanamine in the question stem has one more CH2 than 1-heptanamine in Table 1 and Table 2. According to Table 2, the vapor pressure at 100kPa follows the CH2< in the chemical formula /sub>The number increases and increases, the vapor pressure of 1-octanamine should be greater than 1-heptanamine, choose D