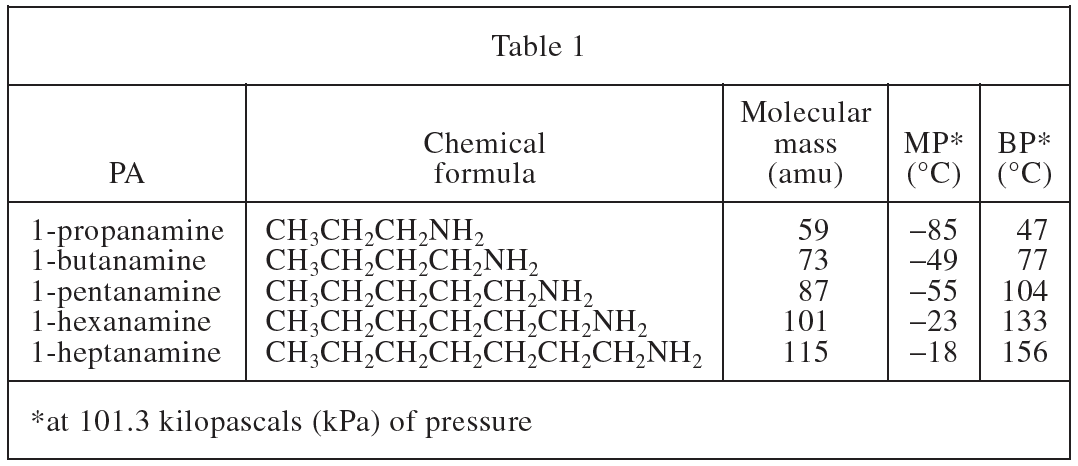
10. Consider the PA ethanamine, CH3CH2NH2. Based on Table 1, the molecular mass of ethanamine is closest to which of the following?
Your Answer is
Correct Answer is G
Explanation
The chemical formulas of the first two PAs in Table 1 differ by one CH2, so the molecule mass of one CH2=73-59=14; the new PA in the question stem One less CH2 than 1-propanamine, so the molecule mass of ethanamine=59-14=45