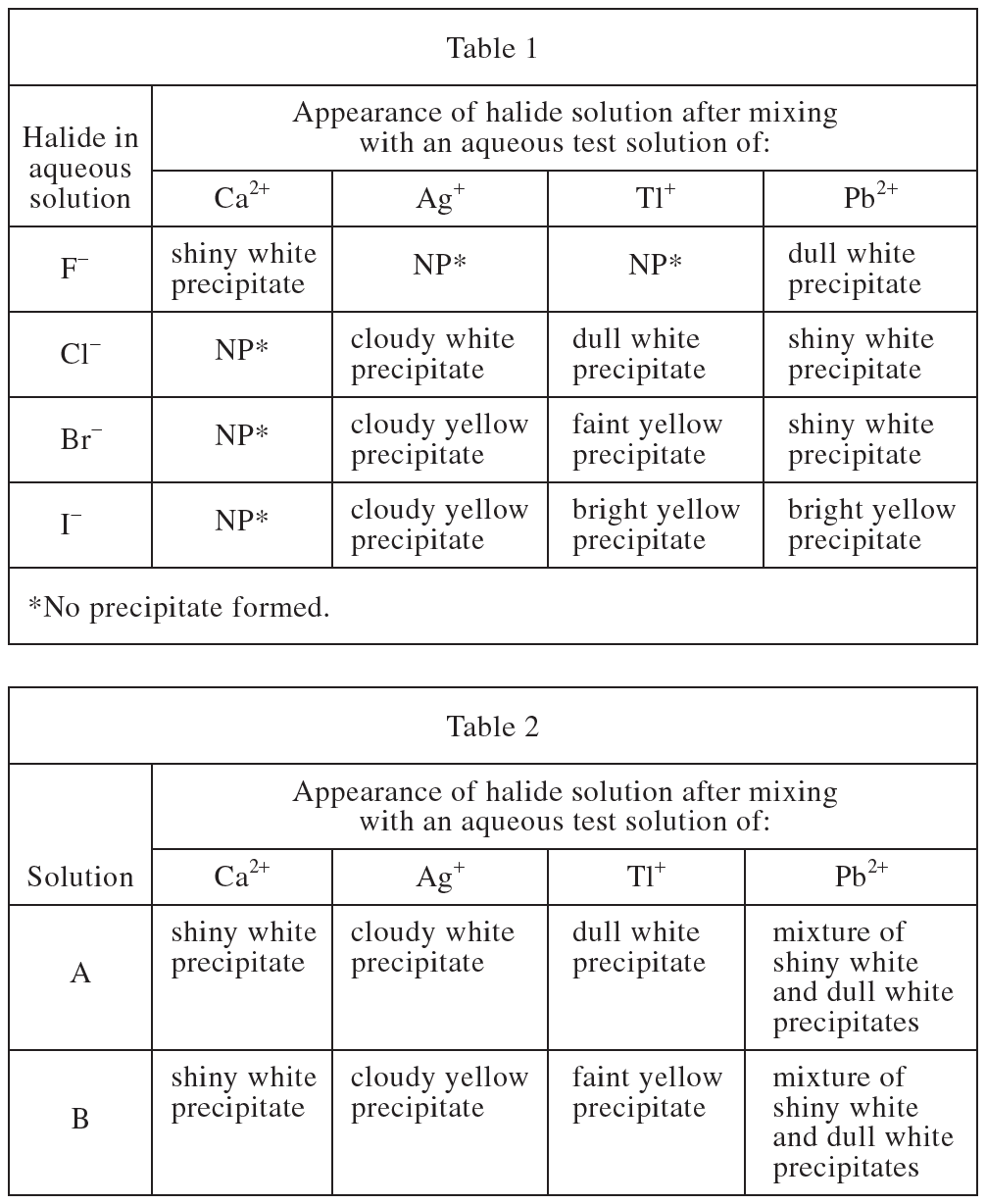
6. When each of the 4 halide solutions is mixed with the Ca2+ test solution, only 1 precipitate forms. This precipitate most likely has what chemical formula ?
Your Answer is
Correct Answer is H
Explanation
For the compound, it is ultimately uncharged. Ca2+ needs two electrons, and F- has only one electron, so the stable structure is CaF2 sub>