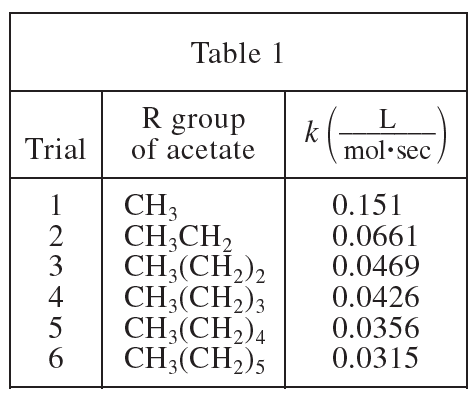
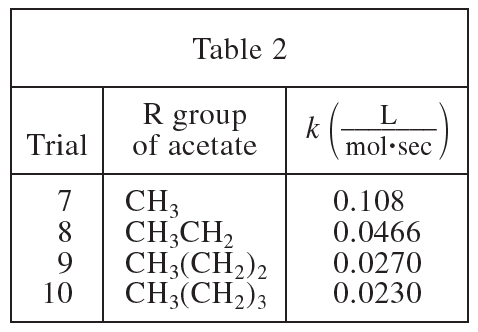
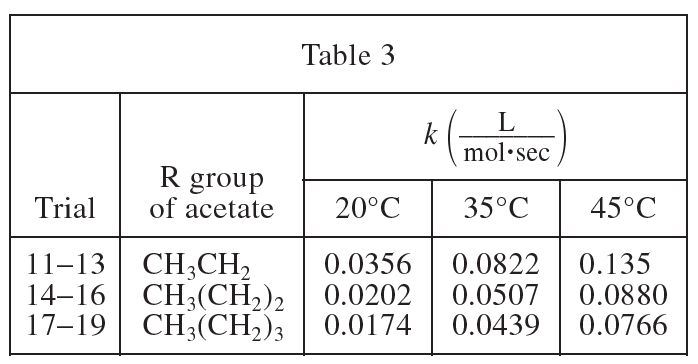
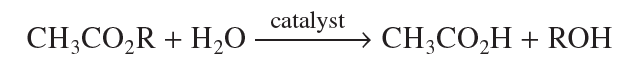26. The dielectric constant (ε) of a substance is a measure of the polarity of the substance; the greater the value of ε, the greater the polarity. If the ε of acetone is 21 and the ε of H2O is 80, was the solvent used in Experiment 1 less polar or more polar than the solvent used in Experiment 2?
Your Answer is
Correct Answer is J
Explanation
According to the question stem information, it can be seen that the polarity of H2O is larger than that of acetone, so the larger the proportion of H2O in the total liquid, the greater the polarity. According to Experiment 1& 2 The text field description shows that H2O accounts for more in Experiment 1, so choose J