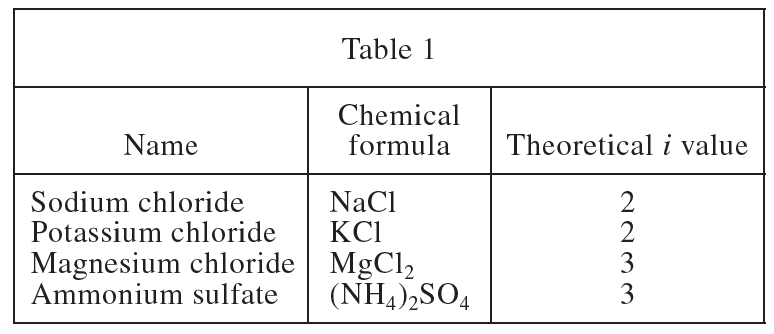
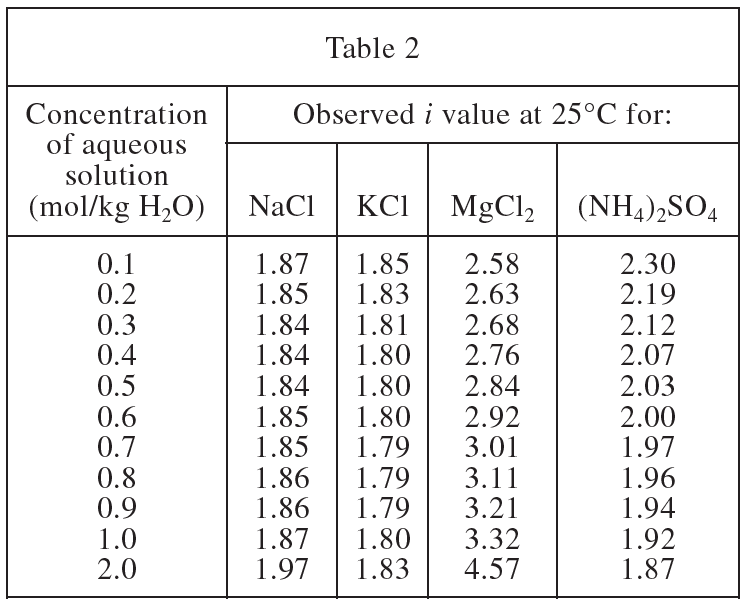
4. Based on Tables 1 and 2, which ionic compound has the largest deviation from its theoretical i value at a concentration of 2.0 mol/kg H2O ?
Your Answer is
Correct Answer is H
Explanation
This question needs to subtract the absolute value of the value in Table 2 when the H2O unit is 2.0 from the value in Table 1 to obtain the deviation of each chemical component. NaCl is |2 -1.97|=0.03, KCl is |2-1.83|=0.17, MgCl2 is |3-4.57|=1.57, (NH4)2< /sub>SO4 is |3-1.87|=1.13, so the largest deviation is MgCl2