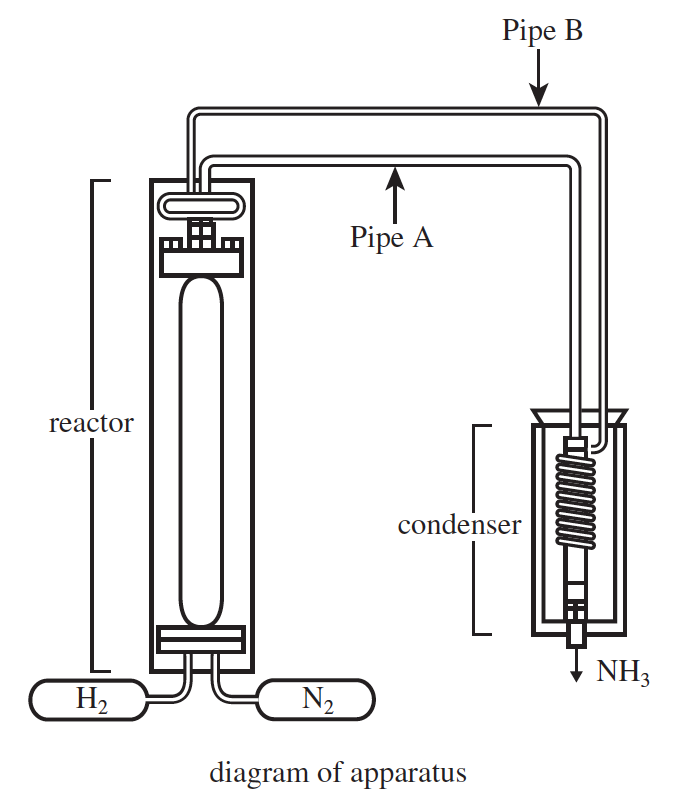
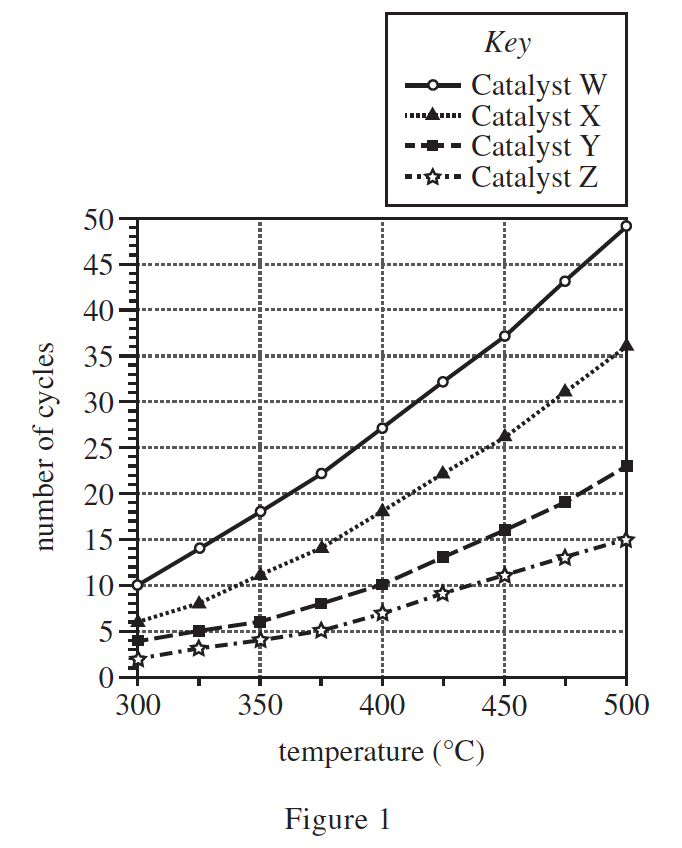
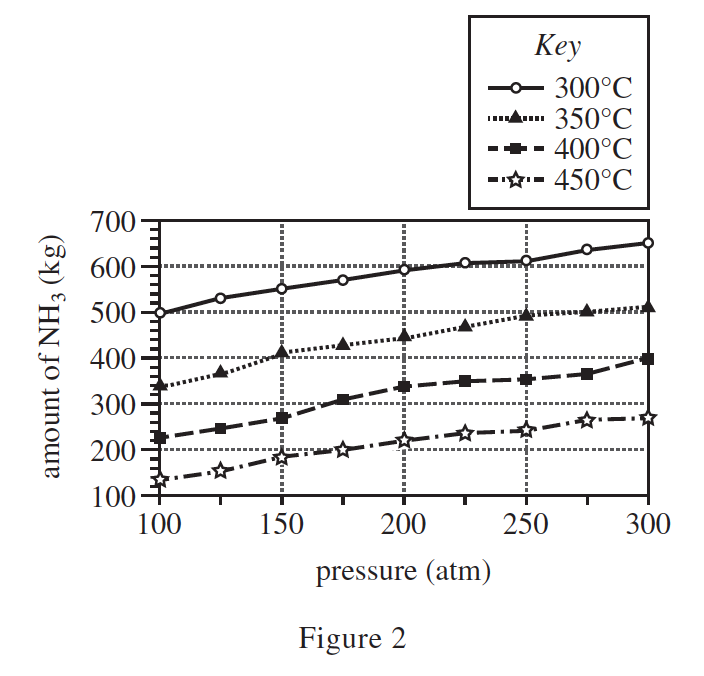
26. At 1 atm of pressure, the melting point of NH3 is −77°C and the boiling point of NH3 is −33°C. Based on this information and the description of the apparatus, when the NH3 exited the condenser, was it more likely a solid or a liquid?
Your Answer is
Correct Answer is J
Explanation
General knowledge questions. Look at the third step of the experimental procedure. The temperature of the condenser is -50 degrees, which is between the boiling point and the melting point, so it is in a liquid state.