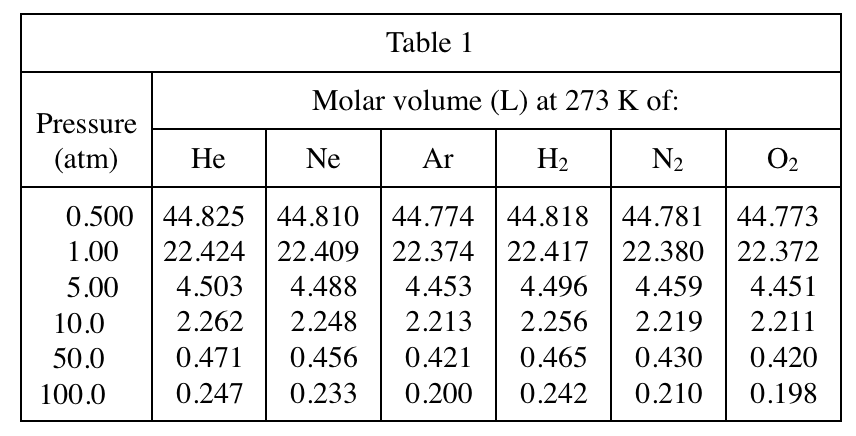
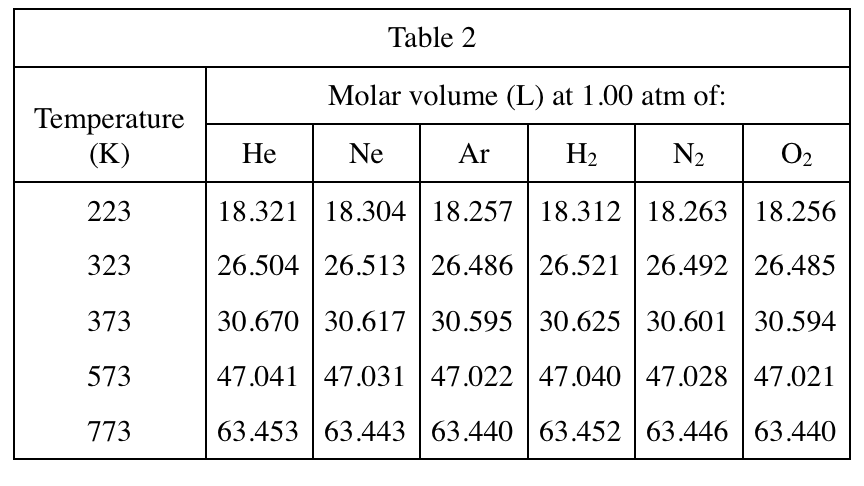
6. Consider 2 separate 1 mol samples of O2, each at a pressure of 1 atm. One sample has a volume of about 18 L, and the other has a volume of about 63 L. Based on Table 2, the average kinetic energy of the O2 molecules is more likely greater in which sample?
Your Answer is
Correct Answer is J
Explanation
Here we need to use a common sense of chemistry, that is, the average kinetic energy of a gas is only related to the temperature. The formula is KE=3/2RT. Looking at Table 2 longitudinally, it is found that the higher the temperature, the larger the volume of the same gas, so vice versa , so the higher the temperature of 63L, the higher the average kinetic energy