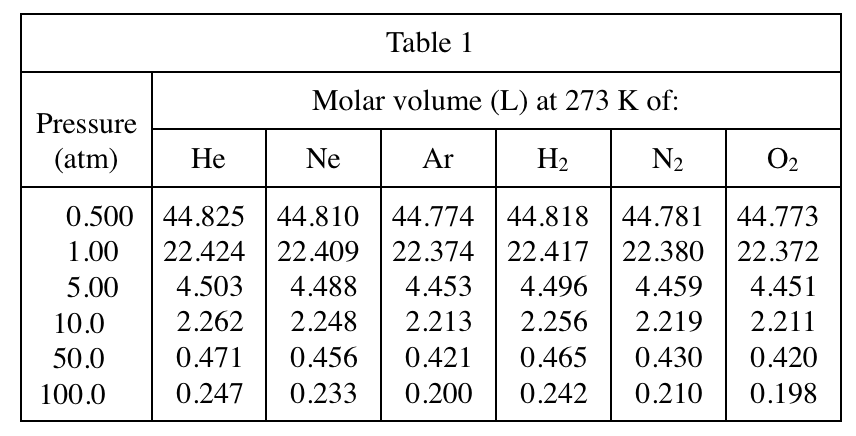
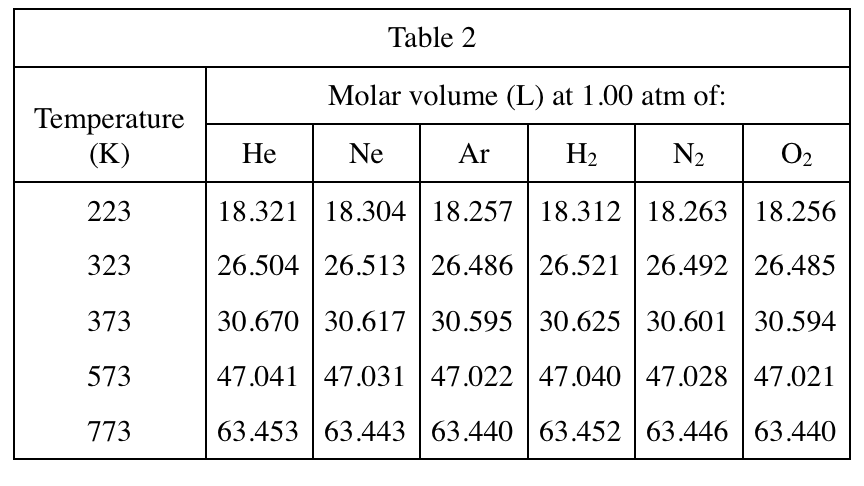
2. Consider the molar volumes of He, Ar, H2, and N2 listed in Table 2 at 323 K. What is the order of these gases from the gas having the smallest molar volume to the gas having the largest molar volume?
Your Answer is
Correct Answer is G
Explanation
Look at Table 2, find the row of 323K horizontally, then compare the molar volumes corresponding to each gas in each column, and then arrange them in order from small to large