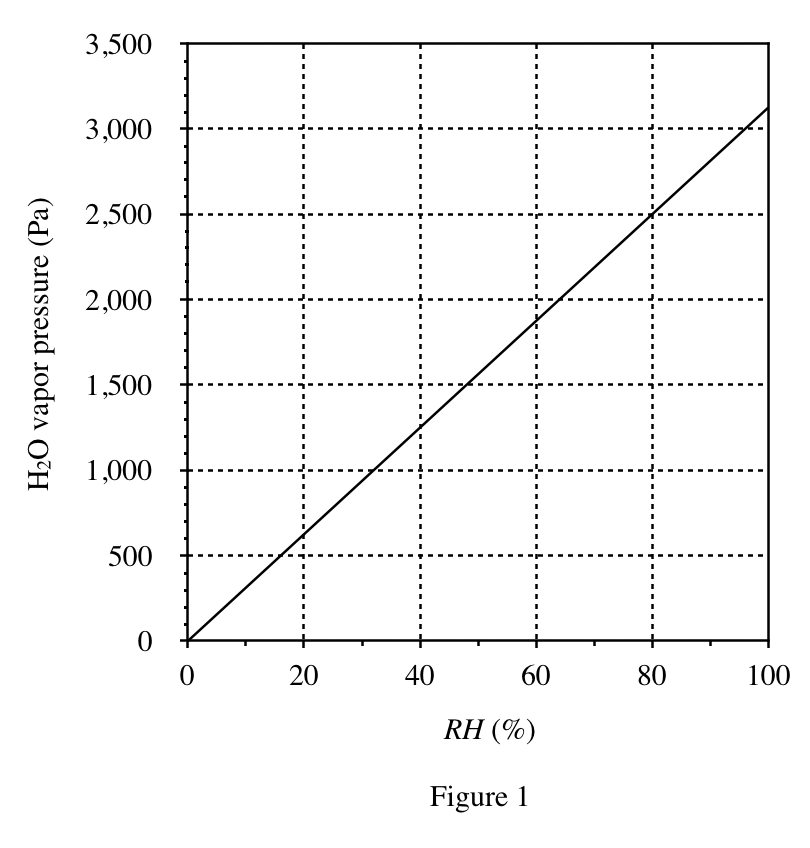
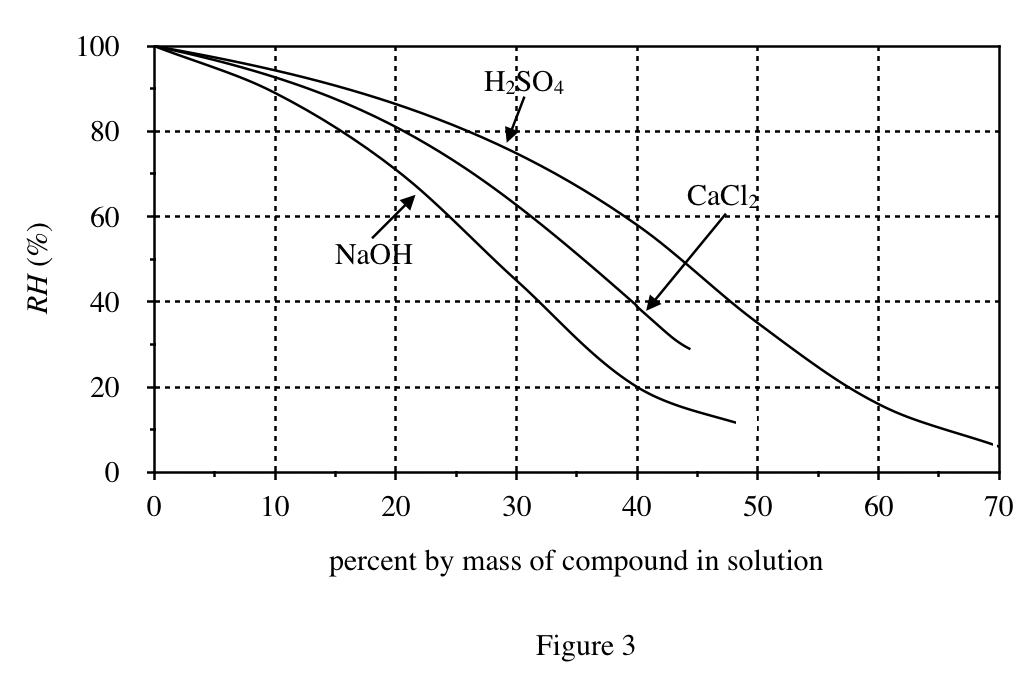
40. Consider two 20% by mass aqueous solutions, one of NaOH and one of H2SO4, each in a separate, closed container. A student claimed that the H2O vapor pressure at CTP will be greater in the air above the H2SO4 solution than it will be in the air above the NaOH solution. Do Figures 1 and 3 support this claim ?
Your Answer is
Correct Answer is J
Explanation
According to Figure 3, the RH of sulfuric acid is higher than that of sodium hydroxide at 20%; the higher the RH, the greater the water vapor pressure.