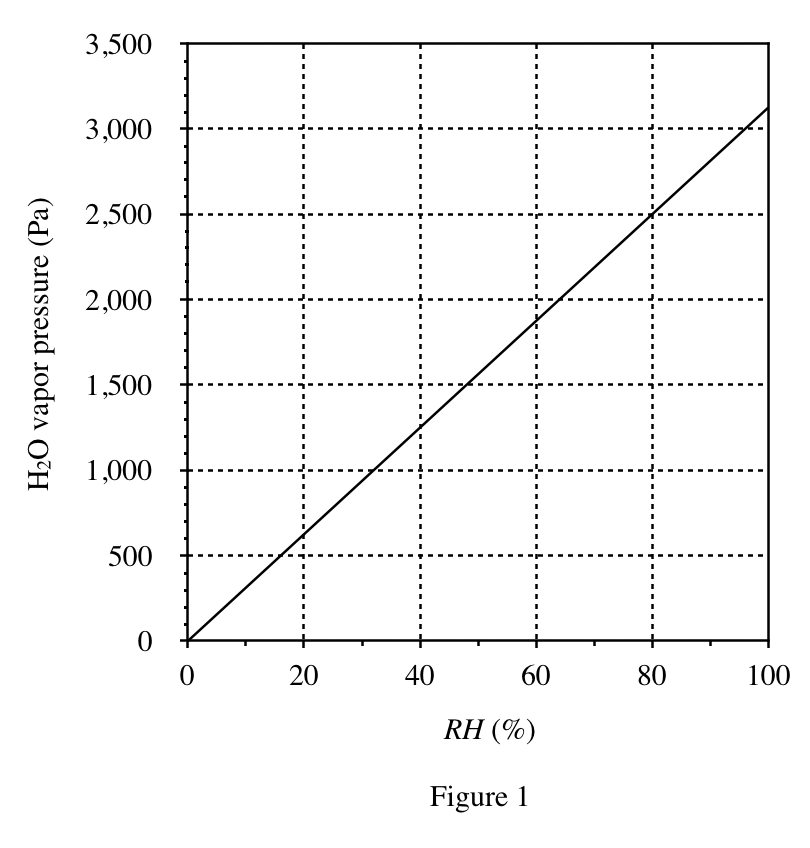
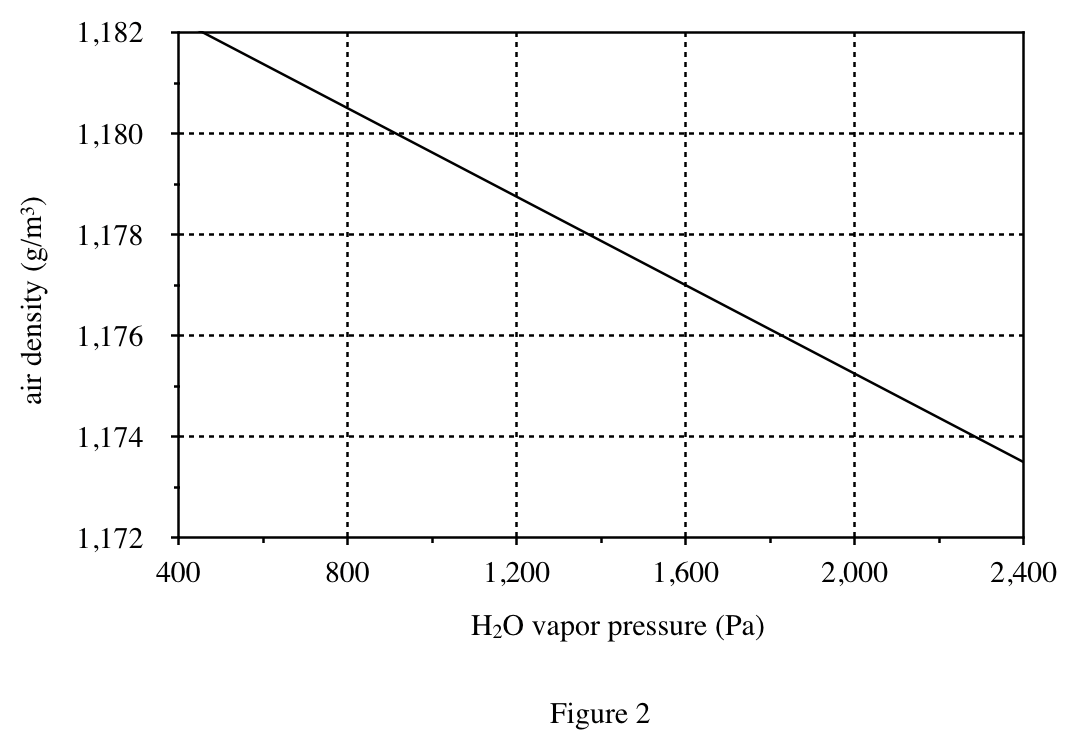
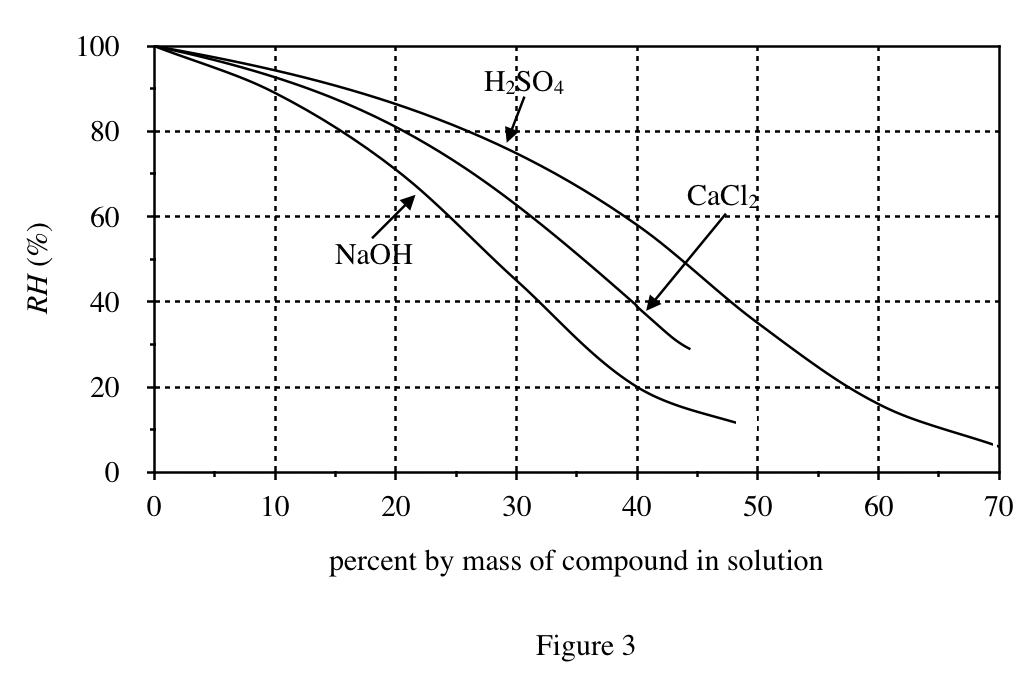
39. Consider a 35% by mass aqueous solution of H2SO4 in a closed container. Based on Figures 1-3, if the air above the solution in the container is at CTP, the density of the air will be closest lo which of the following?
Your Answer is
Correct Answer is B
Explanation
According to Figure 3, when the sulfuric acid concentration is 35%, the RH is about 70%; according to Figure 1, when the RH is 70%, the water vapor pressure is about 2100Pa; according to Figure 2, when the pressure is 2100Pa, the air density is about 1175g /m³.