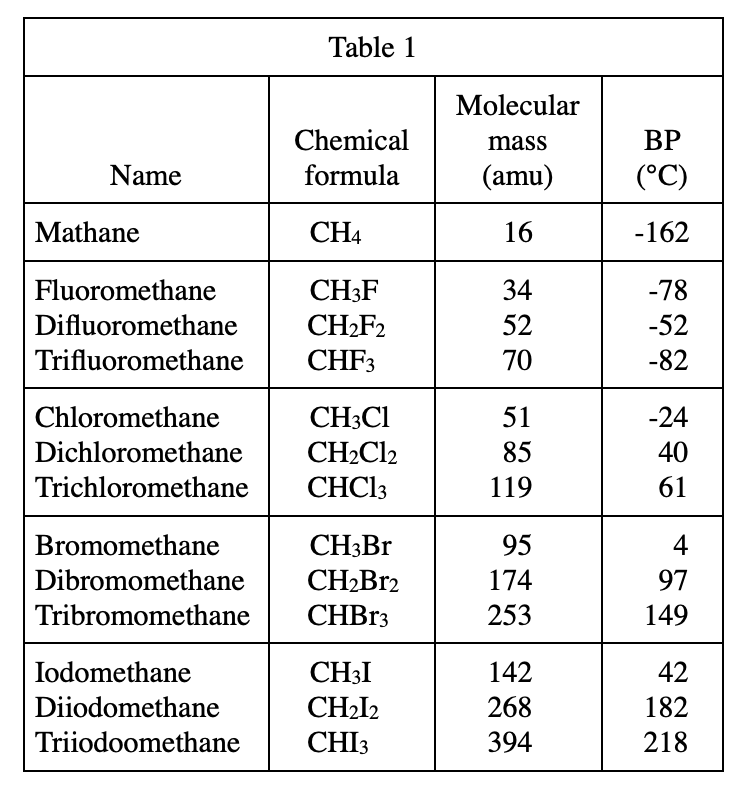
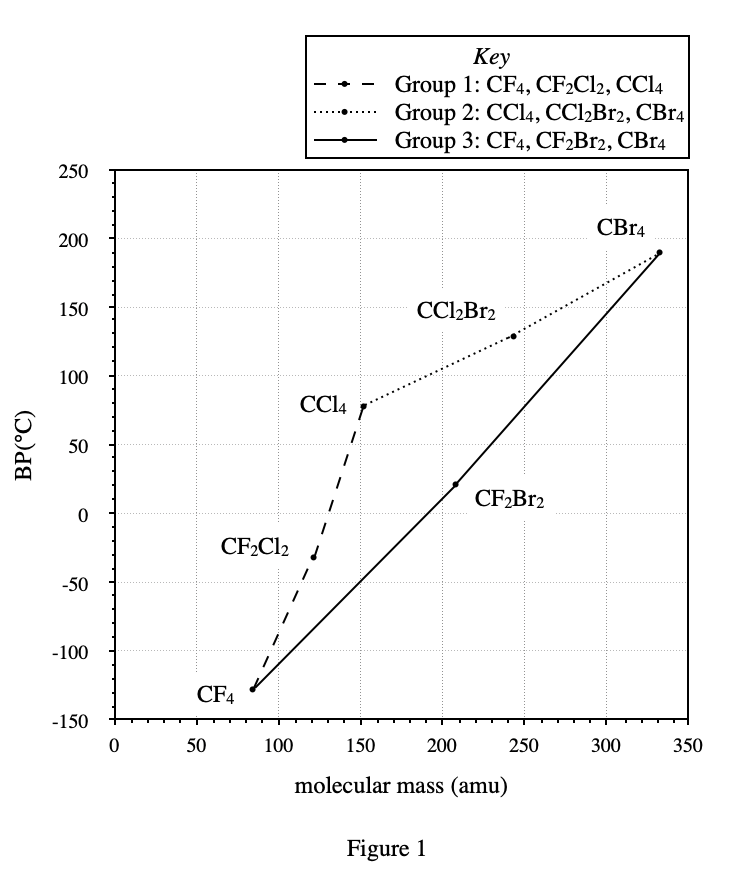
5. According to Table 1 and Figure 1 , the molecular mass of CF4 is closest to the molecular mass of which of the following compounds ?
Your Answer is
Correct Answer is A
Explanation
The atomic mass of an F atom can be calculated through table 1: the molecular mass of CHF3 is 70, the molecular mass of CH2F2 is 52, CH2F2 has one more H atom and one less F atom than CHF3, so the atomic mass of F should be equal to 70 -52+1=19.
So the molecular mass of CF4 should be 70-1+19=88. So according to table 1, the closest molecular mass to CF4 should be chloromethane