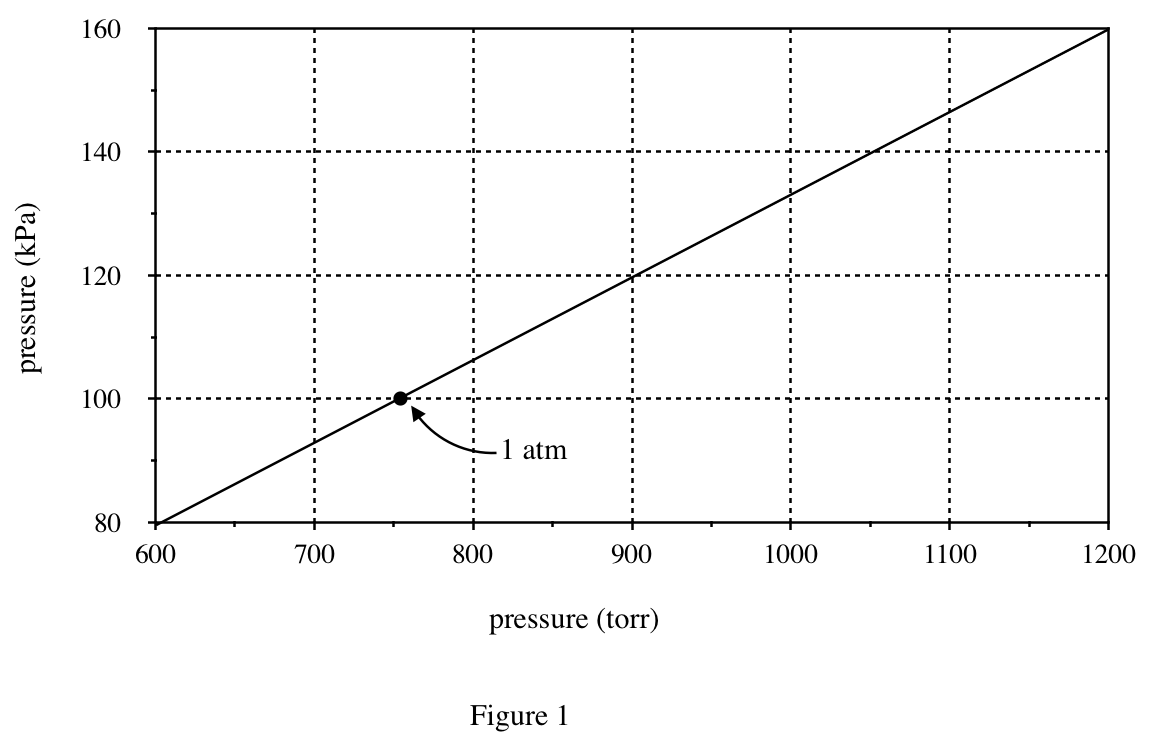
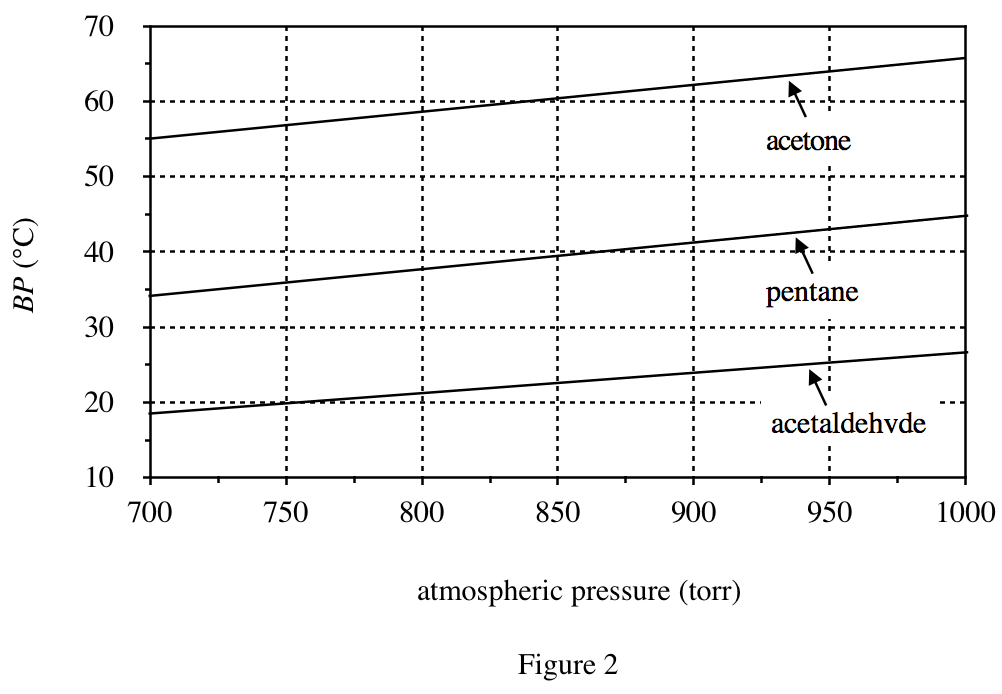
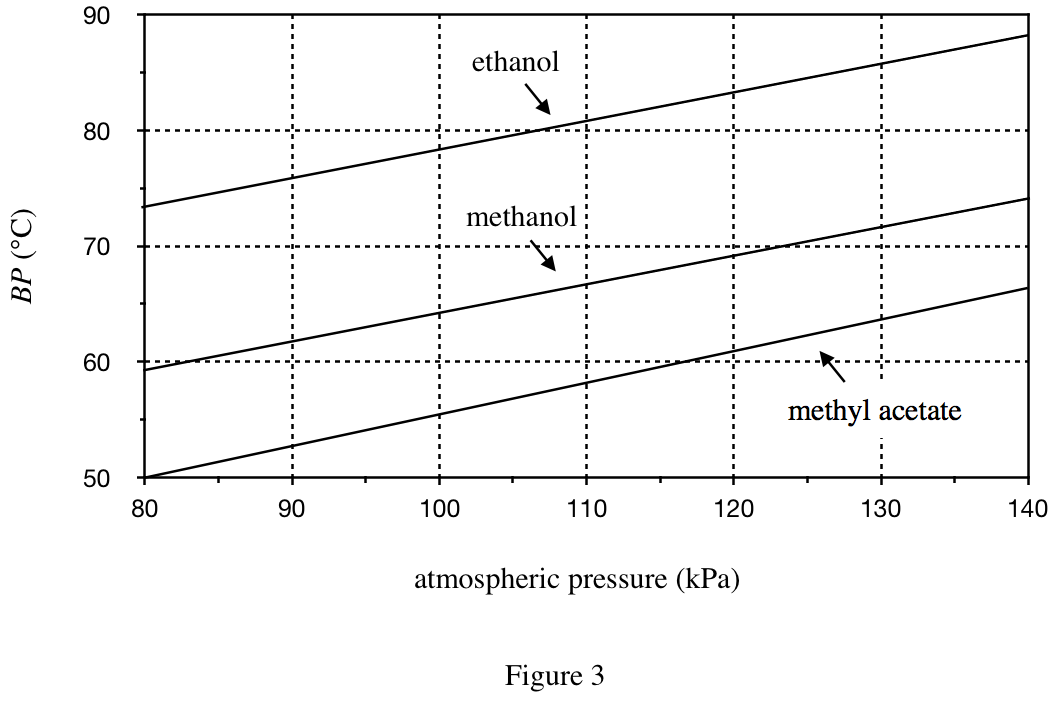
26. Suppose a sample of methanol is in a chamber maintained at 60°C and 110 kPa. Based on Figure 3, if the temperature is kept constant, which of the following changes in the pressure will cause the sample to boil?
Your Answer is
Correct Answer is F
Explanation
From figures 2 & 3, it can be seen that the lower the pressure, the lower the boiling point;
In the case of constant temperature, by changing the pressure, let methanol boil, indicating that the methanol needs to be reduced boiling point, then only by reducing the pressure can the boiling point be reduced to 60°C;
Look at figure 3, when the ordinate BP of methanol is 60°C, the abscissa pressure is 85 kPa, 110-85 =25 kPa, so as long as the pressure is lowered by more than 25 kPa, the boiling point of methanol can be lowered below 60°C, so that methanol boils