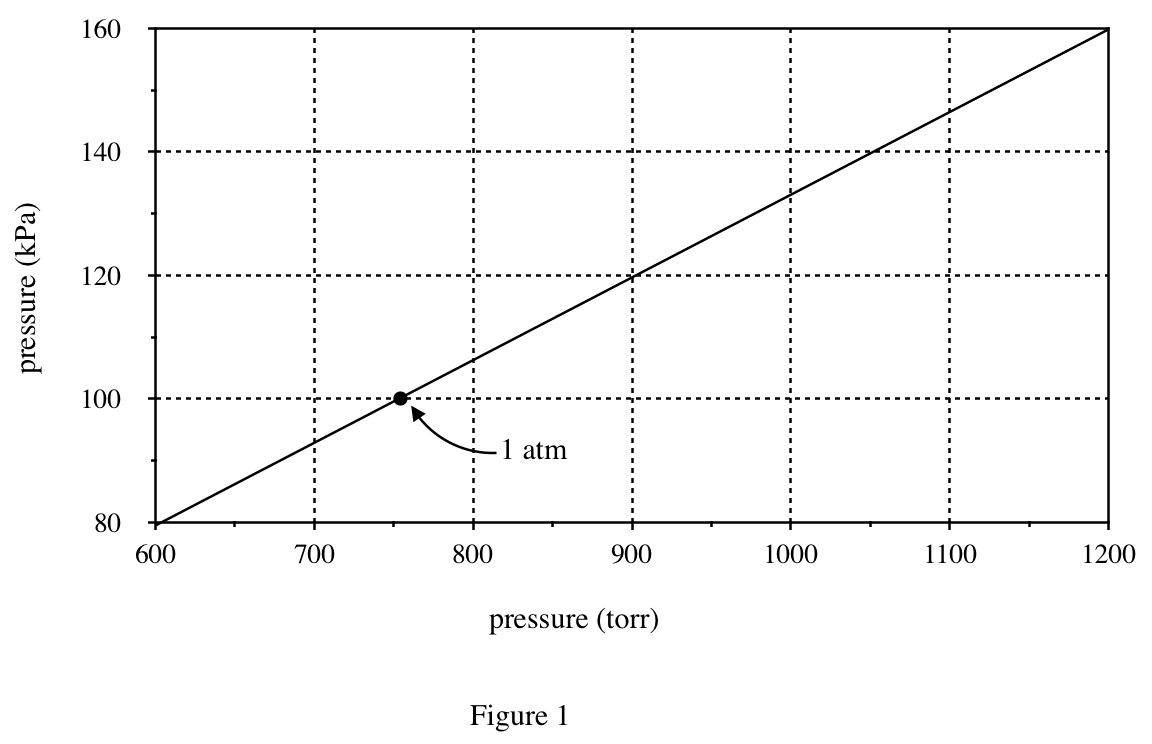
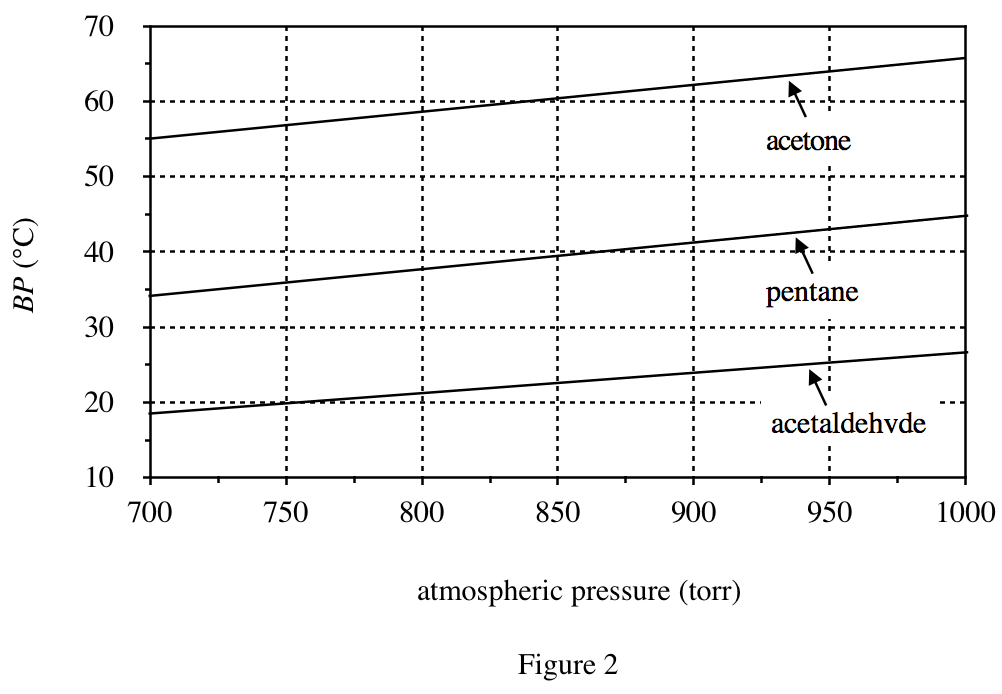
24. A compound’s standard boiling point is the temperature at which the compound boils when the atmospheric pressure is 1 atm. Based on Figures 1 and 2, the standard boiling point of pentane is approximately:
Your Answer is
Correct Answer is G
Explanation
It can be seen from figure 1 that 1 atm is about 760 torr;
Looking at figure 2, when the abscissa is 760, the ordinate of pentane is about 36°C