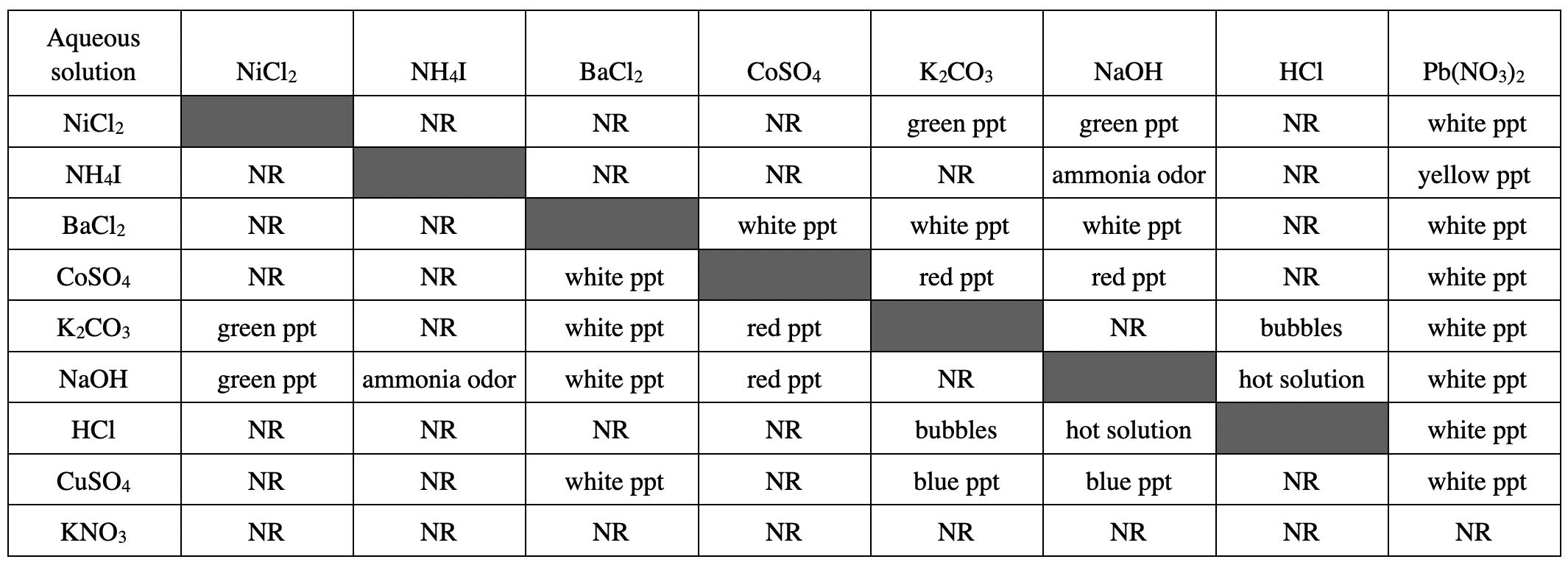
21. An aqueous CoSO4 solution was mixed with 1 of the other solutions listed in the table and a red solid formed. A chemist claimed that the other solution must have contained K2CO3. Based on the table, is his claim valid?
Your Answer is
Correct Answer is A
Explanation
According to the chart, it can be seen that CoSO4 will produce red ppt when encountering K2CO3 or NaOH, so It is incorrect to say that the other reactant must contain K2CO3 in the question stem