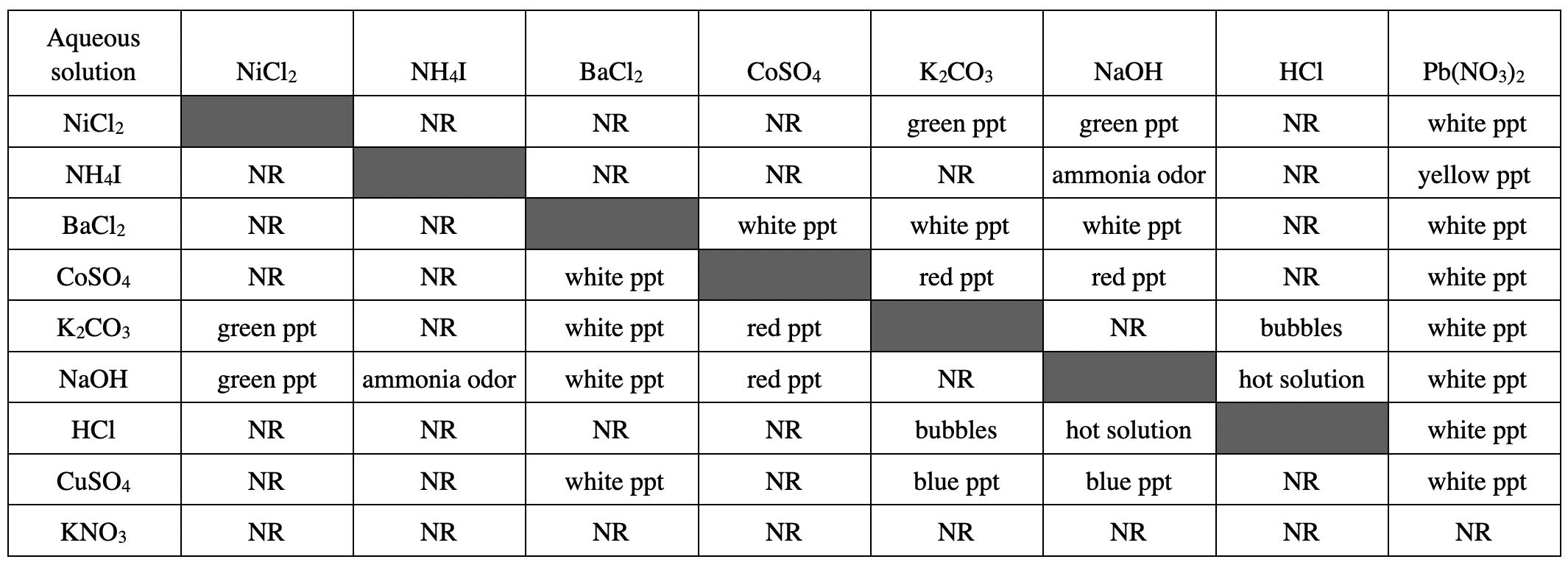
19. A chemist has an unlabeled aqueous solution that contains either NiCl2 or BaCl2. Based on the table, which of the following solutions could be used to determine which of the 2 substances is present?
Your Answer is
Correct Answer is B
Explanation
According to the table, it can be seen that LiCl2+K2CO3 reacts to generate green ppt, while BaCl2+K2CO3 reacts to generate white ppt;
The reactants mentioned in the other options react with LiCl2 Sub> or BaCl2 reaction phenomenon and products can not be seen from the naked eye, so it can not be used to distinguish LiCl2 and BaCl2