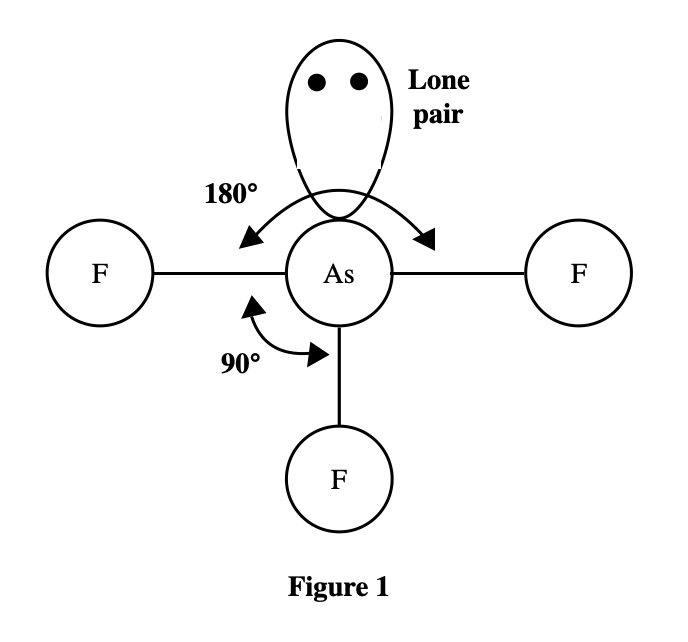
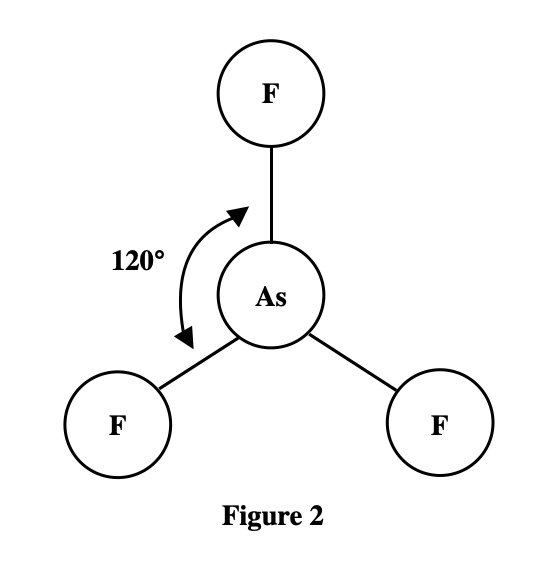
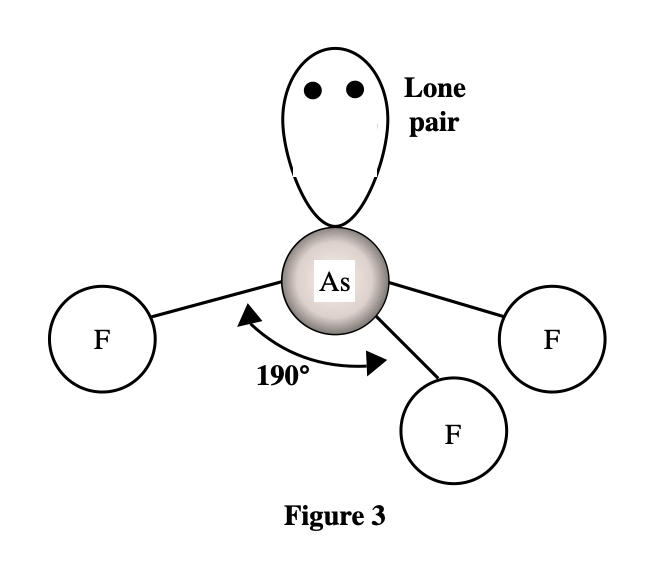
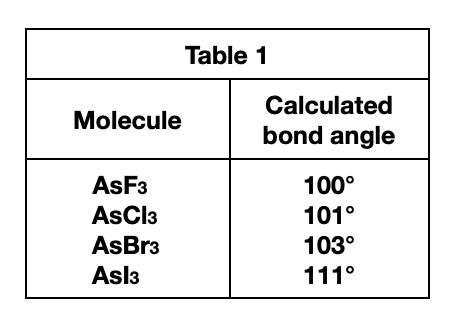
6. A molecule of ammonia () has only 1 unique bond angle, and that bond angle is 107°. The N atom also has a lone pair that strongly repels the 3 N-H bonds. Based on the descriptions given by Students 2 and 3, is the molecular shape of
more likely trigonal planar or trigonal pyramidal?
Your Answer is
Correct Answer is J
Explanation
If the unique bond angle is 107°, then the sum of these angles should be 107°*3=321°, which is not equal to 360°, which means that these atoms are not in the same plane, so it is inconsistent with the statement of student 2, it should be more Close to the trigonal pyramidal of student 3