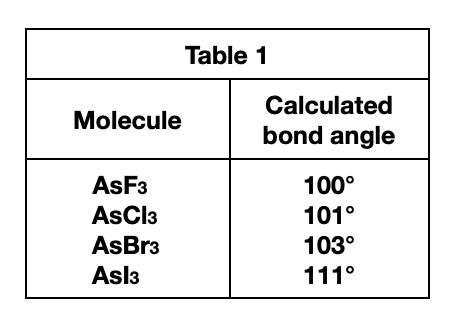
1. The table below gives the atomic mass (in atomic mass units, amu) of the elements F, Cl, Br, and I.
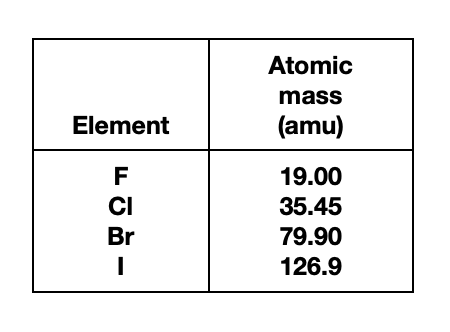
Based on Student 4's description, among the elements listed in the table, as atomic mass increases, atomic radius:
Your Answer is
Correct Answer is A
Explanation
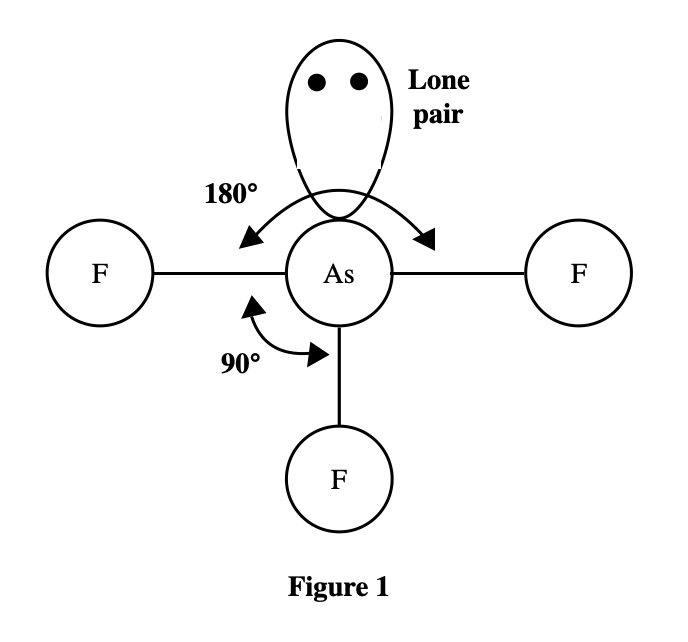
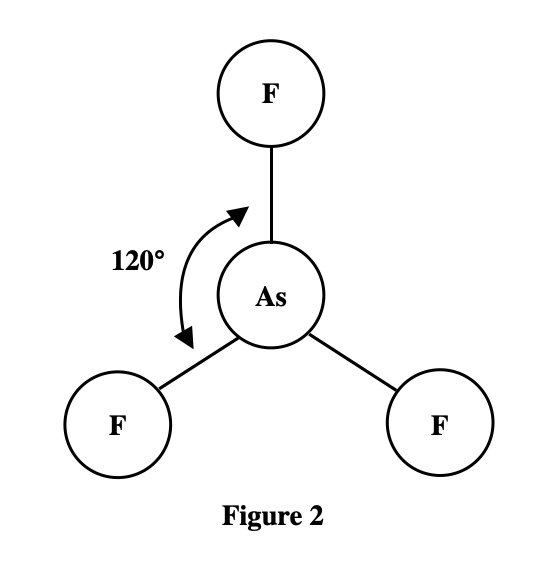
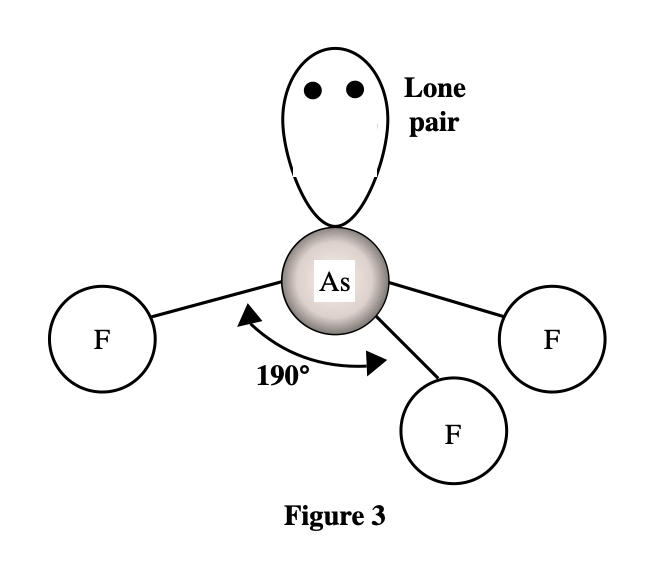
See the larger the atom that is bound to the As atom, the larger the bond angle. The atoms ……size from the smallest to largest, are F, Cl, Br, and I . It can be seen that the atomic radius of I is the largest. According to the table given in the title, it can be seen that the larger the atomic mass, the larger the atomic radius.