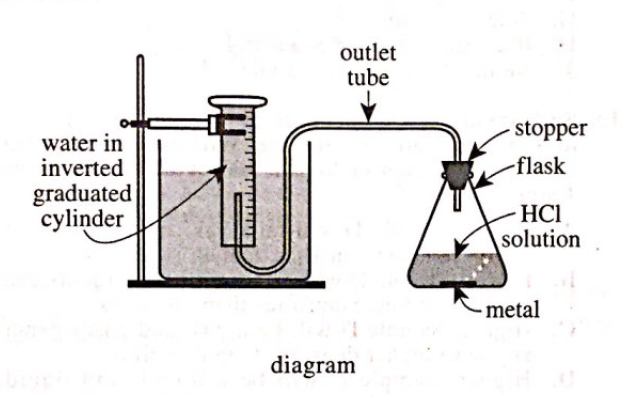
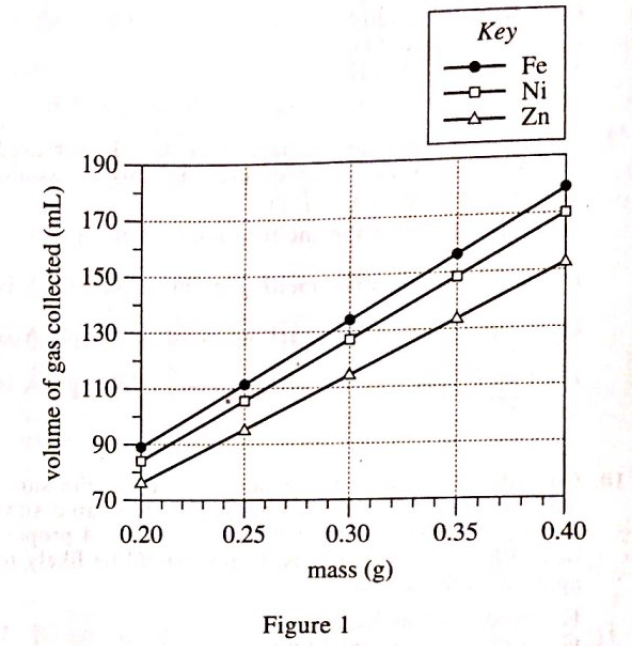
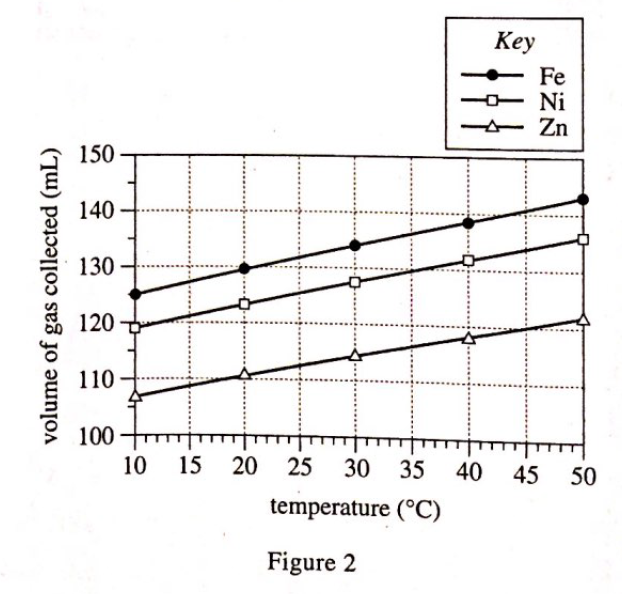
19. Consider the balanced chemical equation in the passage. Based on this equation, if 10 moles of HCl are consumed, how many moles of H2 are produced?
Your Answer is
Correct Answer is A
Explanation
Look at the chemical reaction formula in the article, M + 2HCl→MCl2 + H2, then it is 5M + 10HCl→5MCl2 +5 H2, so when there are 10 mole HCl, there will be 5 mole hydrogen generated