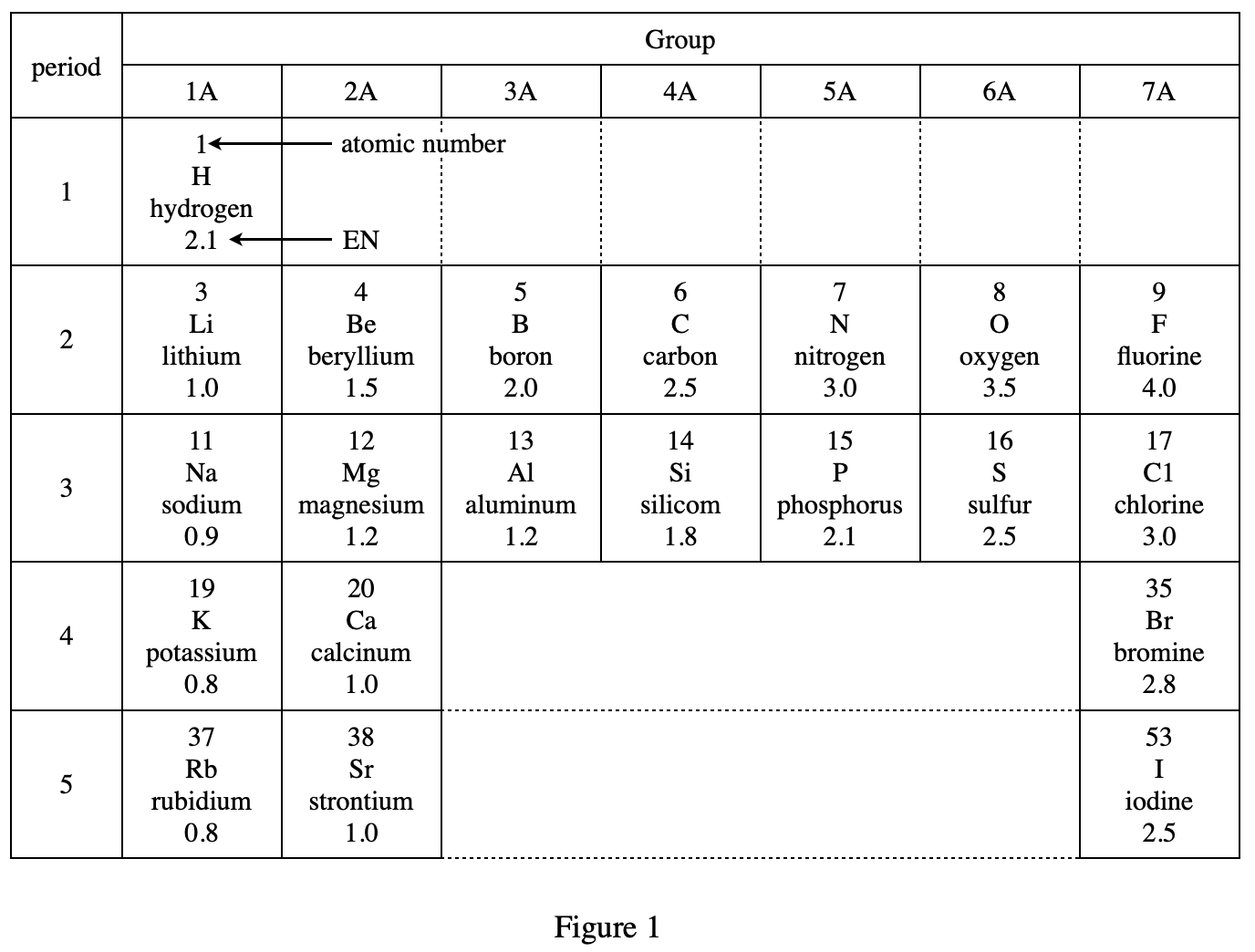
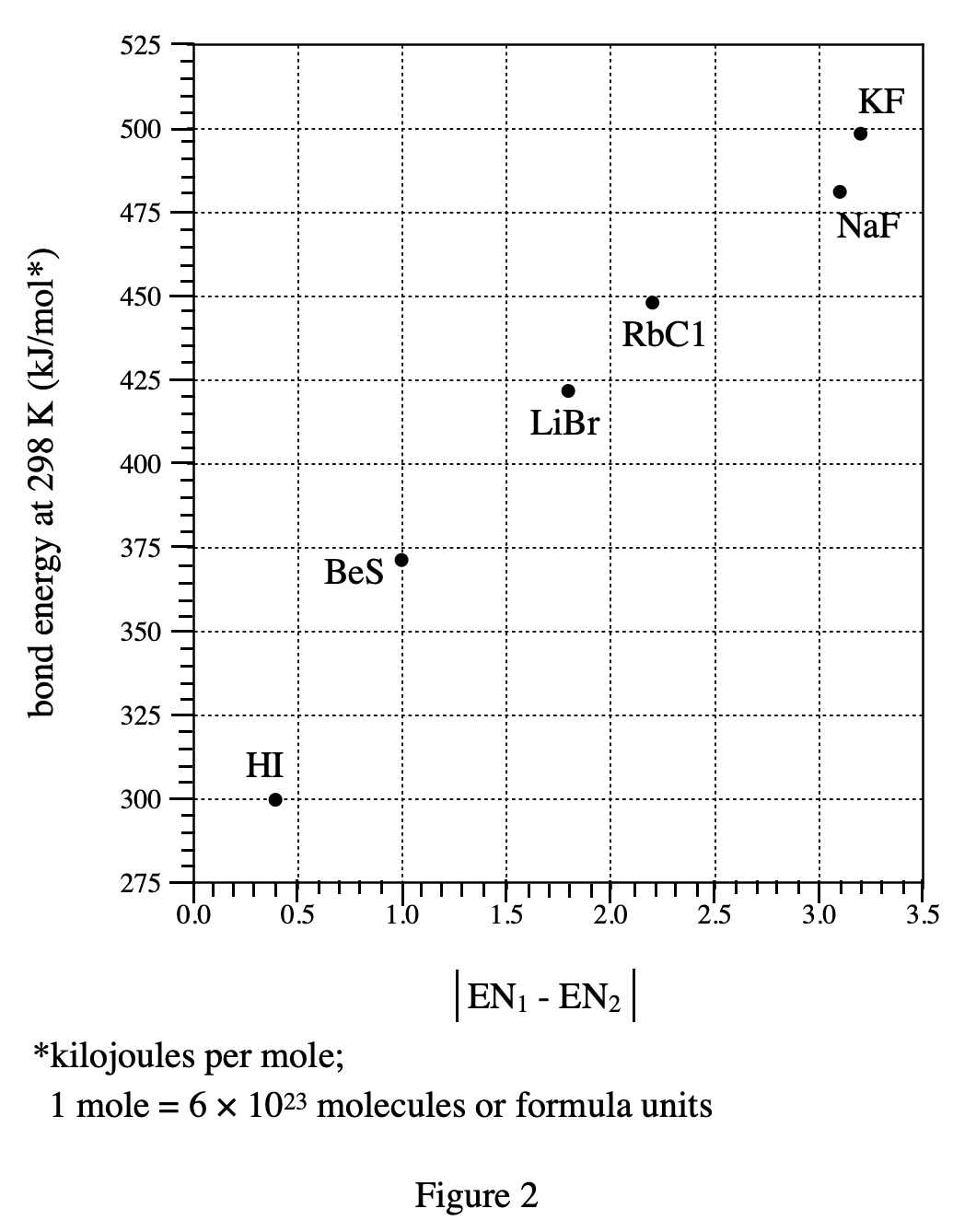
5. Consider | EN1 - EN2 | in Figure 2 for BeS. Based on Figure 1, is the bond in HBr less polar or more polar than the bond in BeS?
Your Answer is
Correct Answer is A
Explanation
Look at the third line of the paragraph above figure 2. The greater the abosolute value of the difference between ENs of the 2 elements, the more polar is the bond between atoms of the elements. From figure 1 we can see H and Br The EN difference between these two elements should be 2.8-2.1=0.7, and from figure 2 we can see that the EN difference of BeS is 1.0, so HBr should be less polar