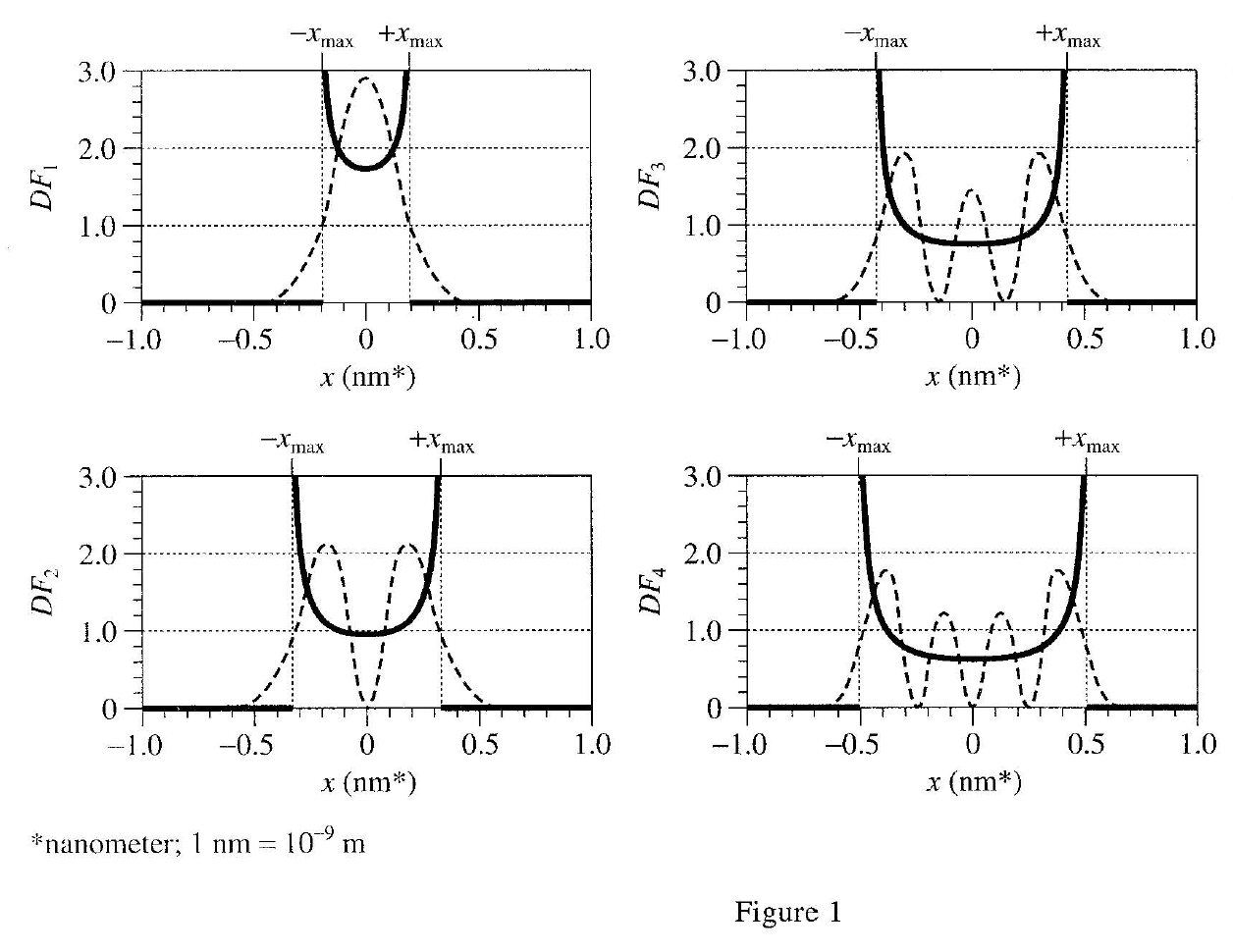
39. Each Quantum DF represents a unique physical state of the electron. The electron may make a transition from one state to another by either absorbing or emitting energy. Based on Table 1, if the electron were initially in the state represented by DF3 and then made a transition to the state represented by DF1 would the electron more likely absorb energy or emit energy, and how much? The electron would:
Your Answer is
Correct Answer is C
Explanation
According to table 1, the electron energy of DF3 is 5, and the electron energy of DF1 is 1, so the energy reduction should be emit energy, which releases 5 -1=4 eV energy