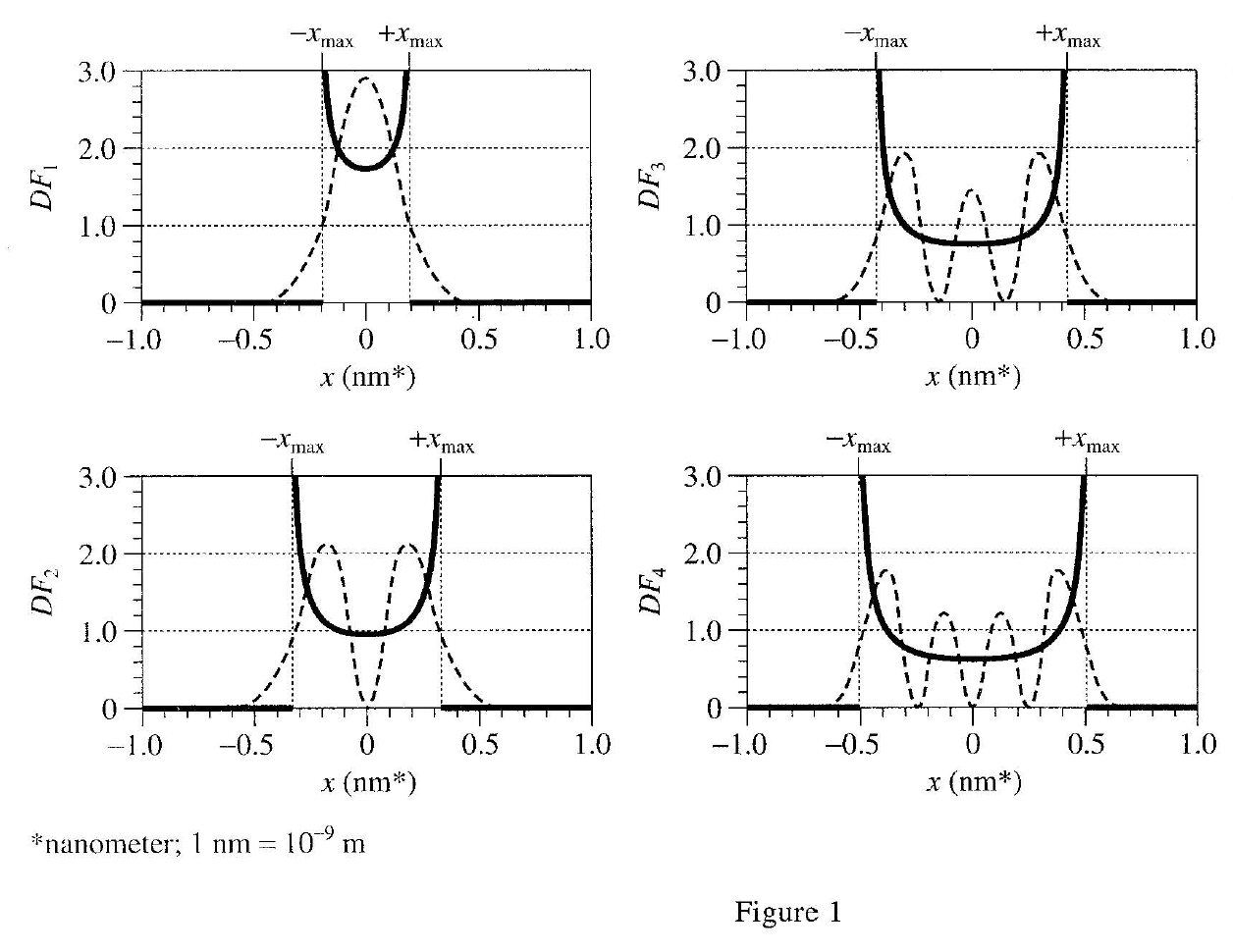
35. The figure below shows the Quantum DF for an electron bound within a hydrogen atom.
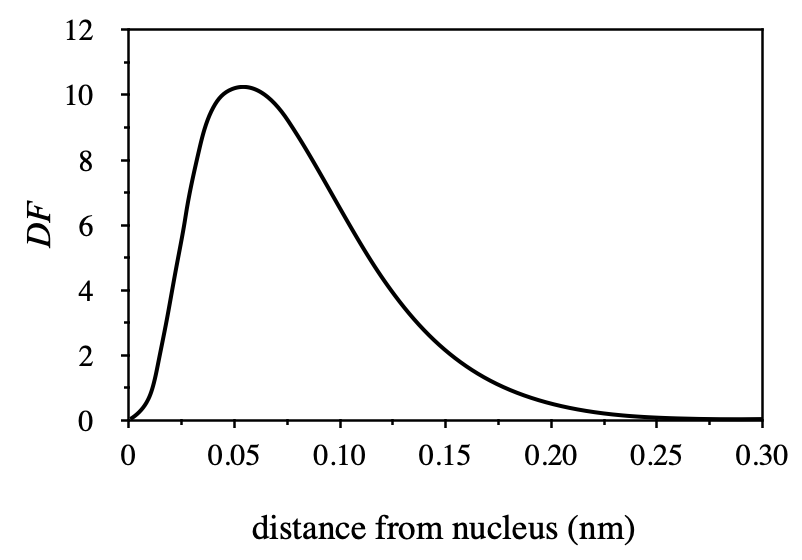
At approximately what distance from the nucleus is this electron most likely to be found?
Your Answer is
Correct Answer is B
Explanation
According to the fourth line of the article, DF stands for electron's energy, so when DF is the largest, the energy of electron is the largest, and electron is most likely to appear at this time. According to the picture in the title, it should be the abscissa corresponding to the peak, which is about 0.05nm