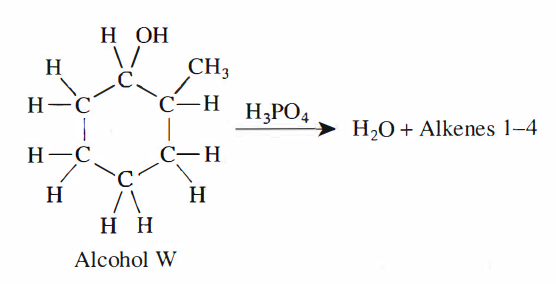
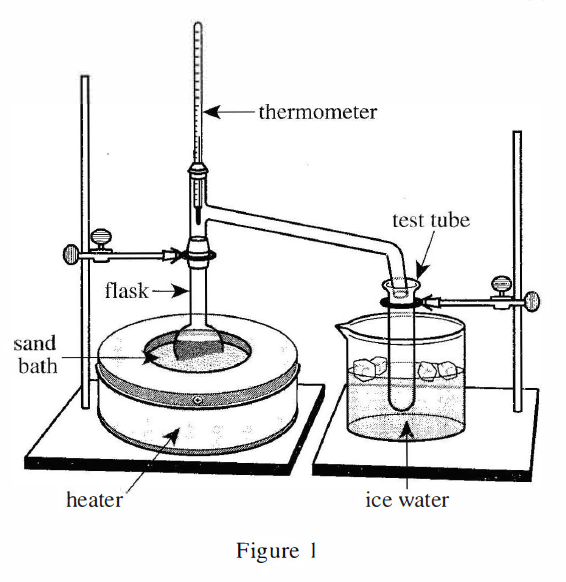
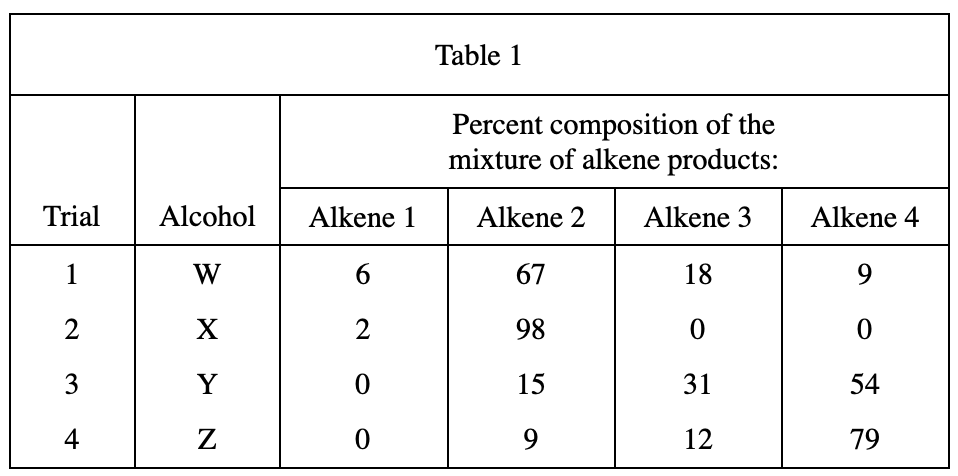
27. One of the students predicted that in Step 3, H2O would be one of the substances collected in the test tube. Based on the boiling point of H2O at 1 atm of pressure and the description of Step 3, was the student’s prediction correct?
Your Answer is
Correct Answer is B
Explanation
General knowledge questions.
Look at step 3 in the article, the heating reaches 115°C, which exceeds the boiling point of water at standard atmospheric pressure by 100°C, so at this time the water will evaporate and become gaseous, and the water vapor will enter along the catheter test tube