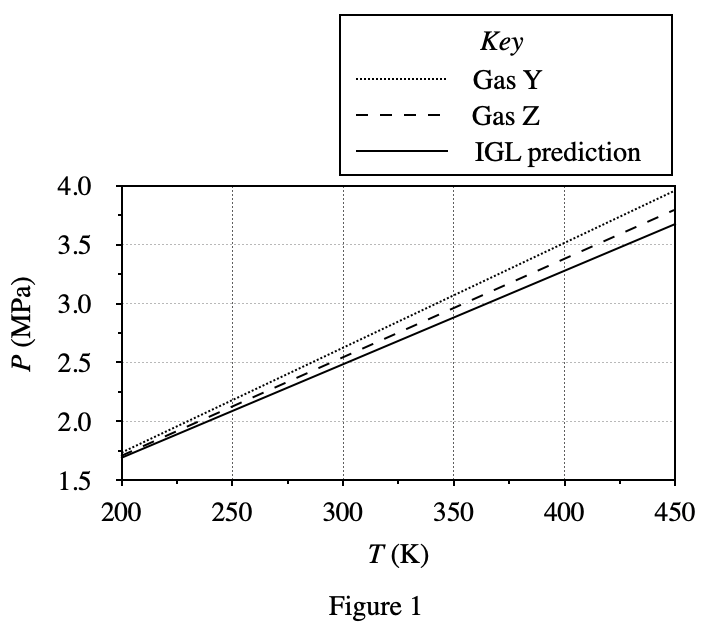
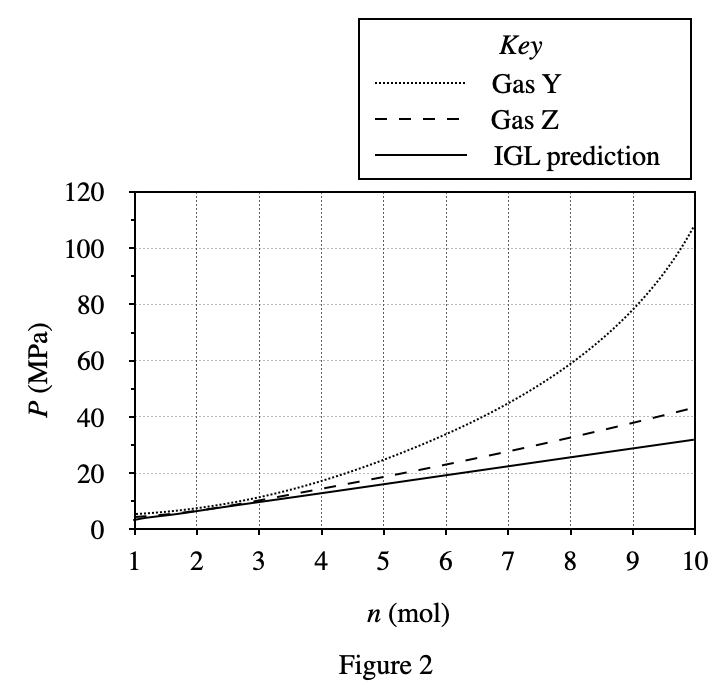
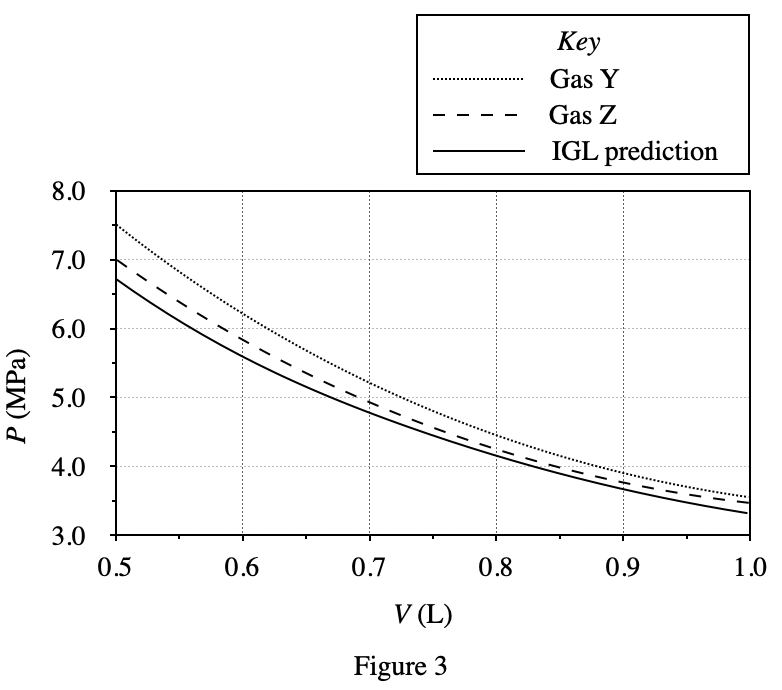
40. Consider an n = 1 mol sample of Gas Z at 400 K in a 1.0 L container of adjustable volume. Suppose the researcher must decrease the container’s volume, but wishes to hold P constant. Based on the results of Experiments 1-3, which of the following additional actions should the researcher perform simultaneously as V is decreased?
Your Answer is
Correct Answer is F
Explanation
It can be seen from figure 3 that the gas pressure will increase as the container volume decreases; and from figure 1 and figure 2, it can be seen that the gas pressure will increase with the decrease of temperature T and mole number n Small and reduced. Therefore, when the volume of the container becomes smaller, the gas pressure will increase. In order to keep the pressure constant, it is necessary to lower the temperature or increase the number of moles of the gas, so only option F is feasible