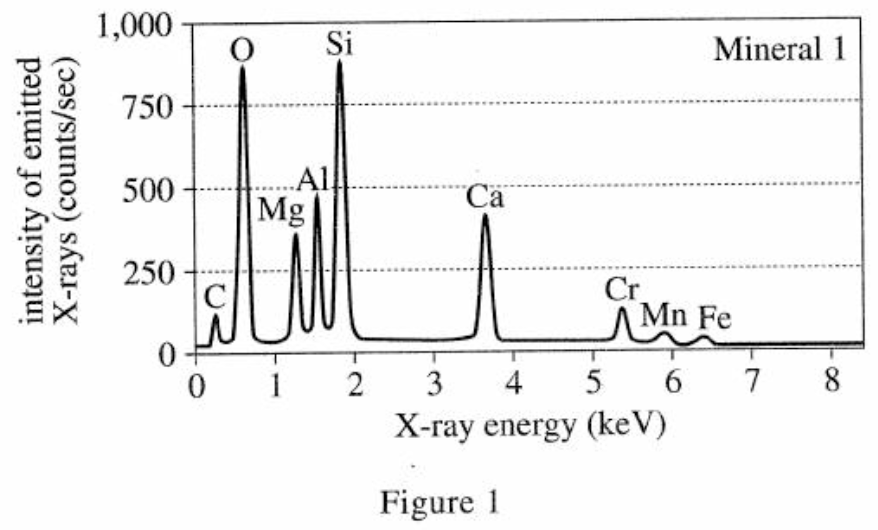
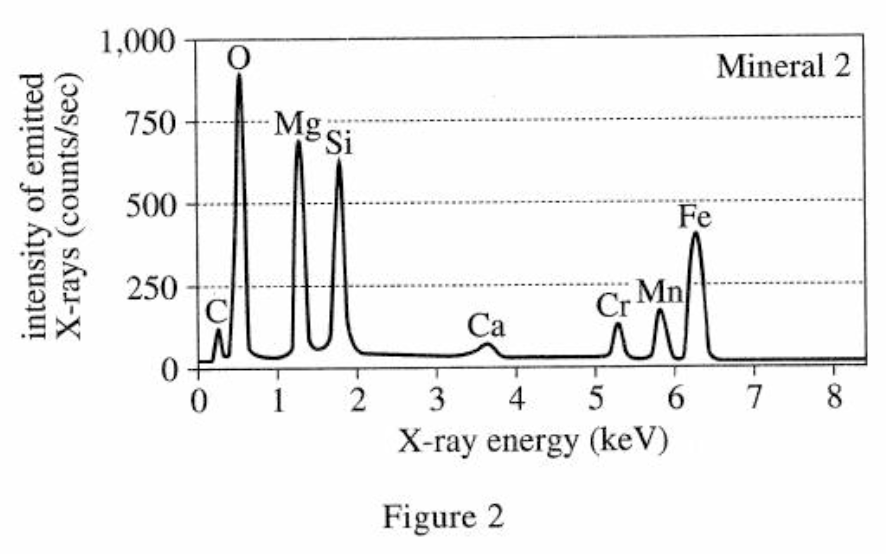
3. The energy of the most intense X-rays emitted by an element is directly related to the element’s atomic number. The atomic numbers of several of the elements are shown in the table below.
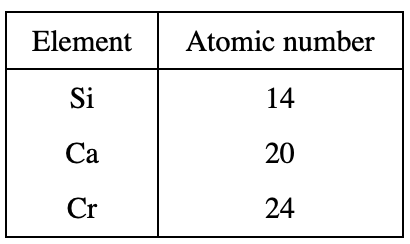
If an element with an atomic number of 22 had been present in Mineral 1, the energy of the most intense X-rays emitted by this element would most likely have been:
Your Answer is
Correct Answer is B
Explanation
The atomic mass of the element mentioned in the question stem is directly related to the intensity emitted. It can be seen from the table in the title that the element with an atomic mass of 22 is between Ca and Cr, so the intensity emitted should also be between Ca and Cr. From figure 1, it can be seen that the energy is in the range of 3.5 keV-5.5 keV