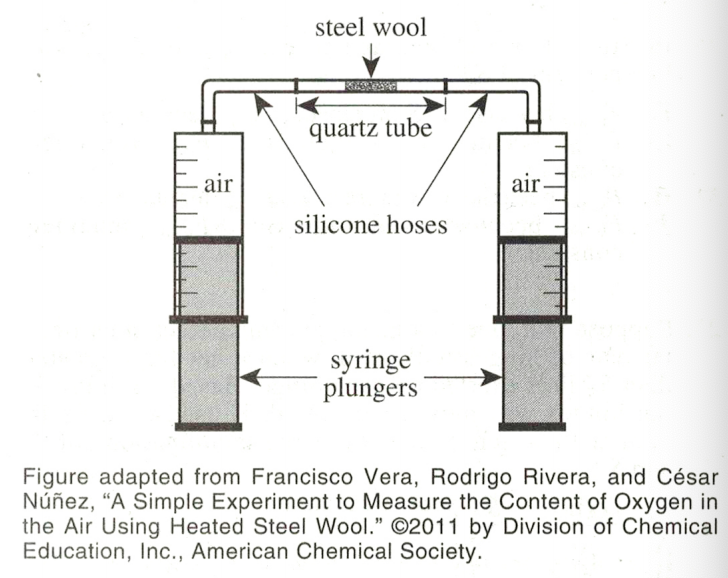
34. In a chemical reaction, the limiting reactant is the reactant that is in the shortest supply and thus limits the amount of product that can be produced. Which student would be the most likely to agree that the limiting reactant during the demonstration was the iron in the steel wool?
Your Answer is
Correct Answer is G
Explanation
Student 2 said in the first two sentences that the reactants are O2 and Fe, and O2 in the air accounts for 80%, but after the final reaction The total volume of the gas only decreased by 20%, indicating that there is a surplus of O2, and the reason for the end of the reaction is that Fe is consumed, so Fe is a limiting reactant