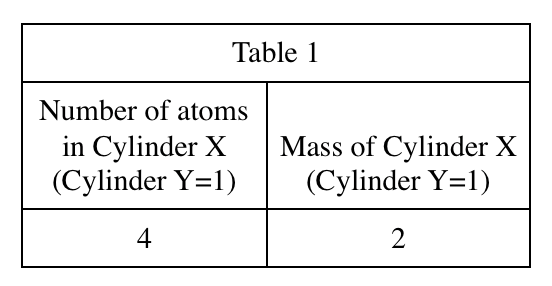
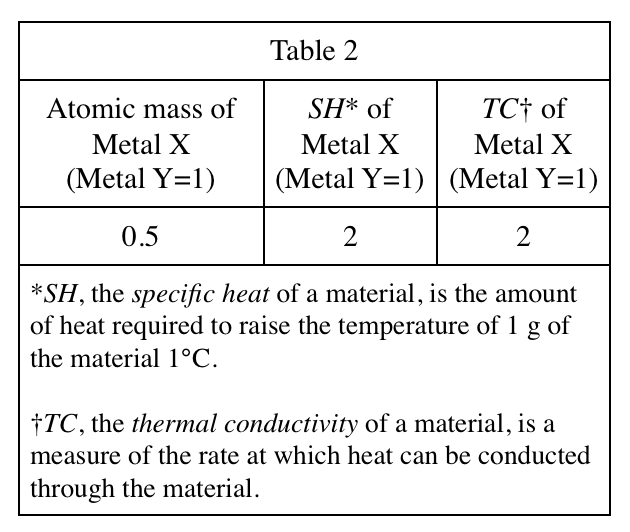
38. A unit of heat capacity is cal/°C. Based on Student 2's definition of heat capacity, which of the following must be a unit of specific heat?
Your Answer is
Correct Answer is H
Explanation
Look at the explanation of SH in table 2, SH is the amount of heat required to raise the temperature of 1 g of the material 1°C, so heat capacity should be divided by mass, which is cal/°C÷g , which is cal/(g °C)