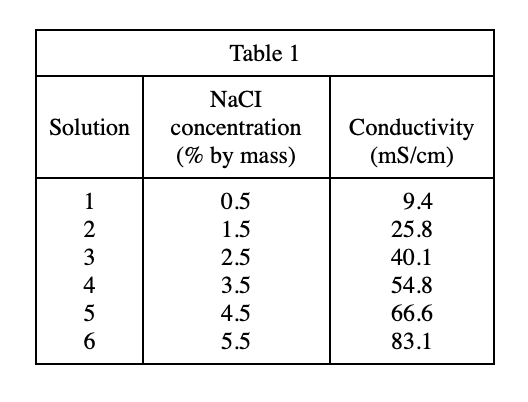
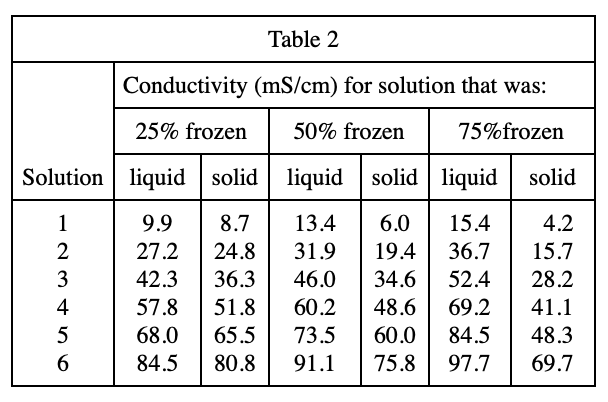
40. Suppose 20 g of Solution 1 was mixed with 20 g of Solution 3 and the resulting solution was tested as in Experiment 1. The conductivity of the resulting solution would most likely be closest to which of the following?
Your Answer is
Correct Answer is G
Explanation
Looking at table 1, the concentration of Solution 1 is 0.5, and the concentration of Solution 3 is 2.5. When the two solutions are mixed in equal amounts, the resulting solution concentration is (0.5+2.5)/2=1.5, which is equal to that of Solution 2. Concentration, so the conductivity should also be equal to the conductivity of Solution 2, which is 25.8, and the G option is the closest