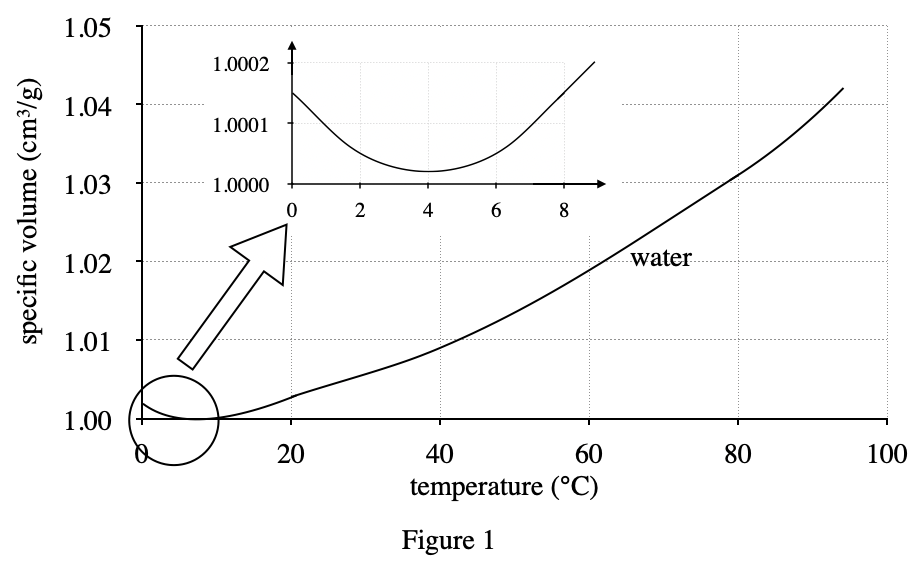
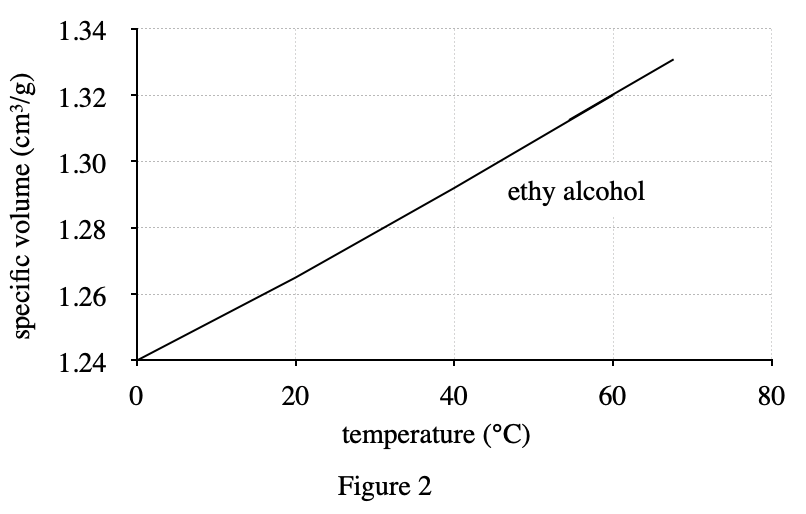
4. Assume that solid ethyl alcohol at its freezing point, -117°C, is denser than liquid ethyl alcohol at 0°C. Based on Figure 2, solid ethyl alcohol's specific volume at -117°C is:
Your Answer is
Correct Answer is F
Explanation
It can be seen from figure 2 that as the temperature on the abscissa decreases, the specific volume on the ordinate also decreases, so when the temperature is -117°C, the specific volume should be lower than 1.24 at 0°C