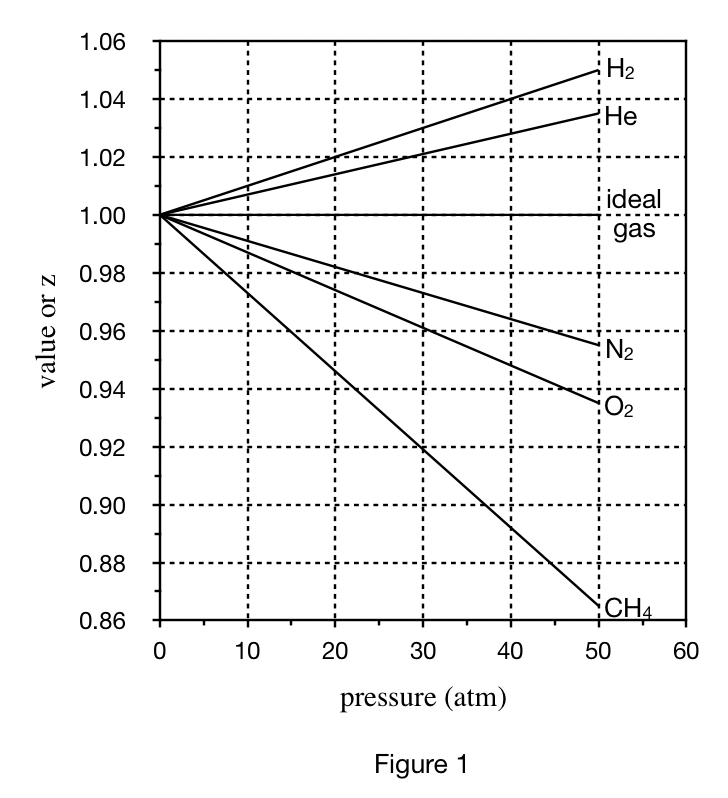
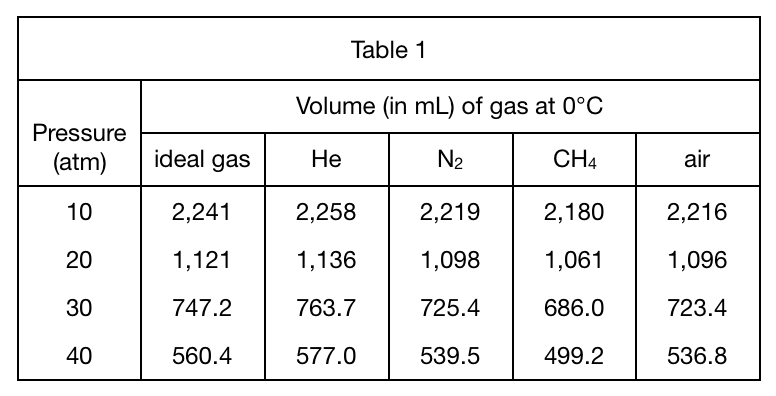
23. Based on Table 1, at 40 atm, the volume of 1 mole of CH4 at a temperature of -30℃ will most likely be:
Your Answer is
Correct Answer is A
Explanation
Look at CH4 in table 1, when the pressure is 40 atm, its volume is 499;
Then because the condition required by the title is -30°C, And the temperature in table 1 is 0°C. Therefore, the lower the temperature, the lower the pressure of the gas, so the volume at -30°C must be less than 499, only the less than 500 mL of option A meets