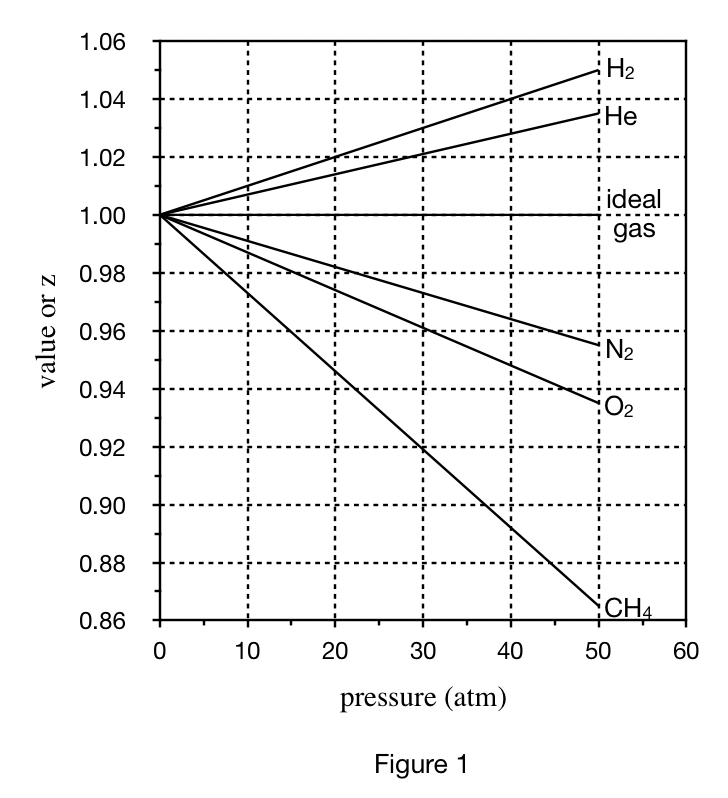
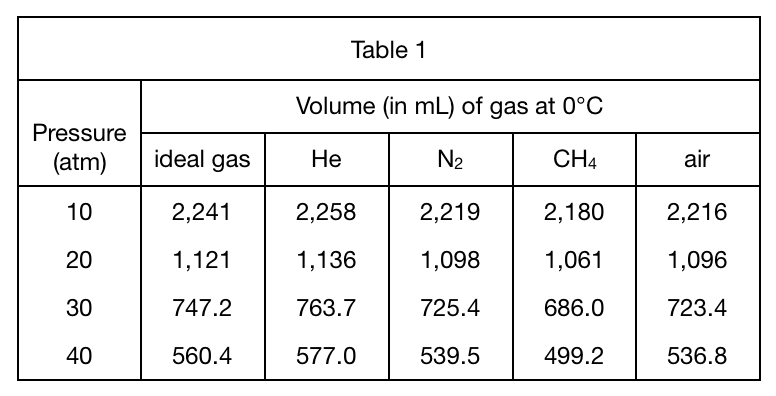
22. Based on Figure 1 and Table 1, at 0°C and 30 atm, the volume of 1 mole of O2 will most likely be:
Your Answer is
Correct Answer is G
Explanation
Look at the line with pressure of 30 atm in table 1 first, and find that the larger the volume of the first four gases, the higher the position of the corresponding line in figure 1;
Look at figure 1 again The O2 curve is between the CH4 and N2 curves, so it is inferred that the volume of O2 is in the CH4 Between 686 of sub>4 and 725.4 of N2