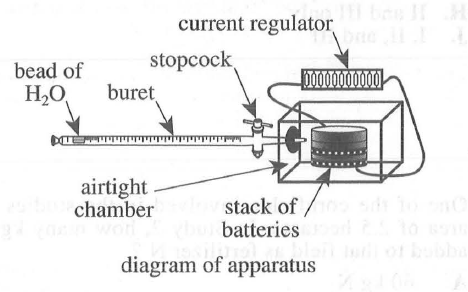
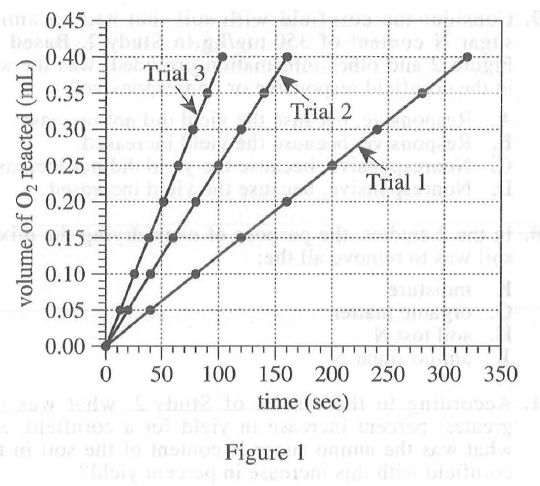
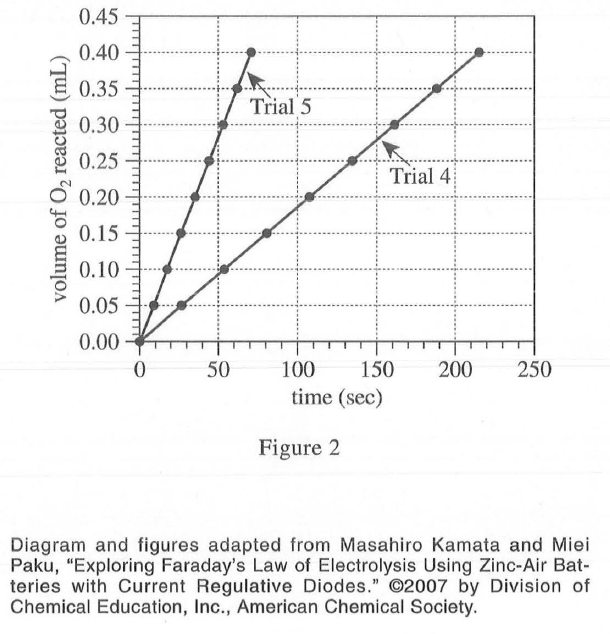
29. Suppose Trial 1 is repeated except at a current of 10 mA. At 200 sec, the volume of O2 reacted will most likely be:
Your Answer is
Correct Answer is A
Explanation
Comparing trail 4 and trail 5 in experiment 2, we can see that the smaller the current intensity, the slower the reaction. Therefore, after seeing the current intensity of 20 mA in trail 1 to 10 mA, the reaction speed of O2 becomes slower, and the reaction volume at 200 sec is smaller than the original 0.25 mL