35. The nonpolar solid 9, 10-diphenylanthracene has a molecular mass of 330 amu and readily dissolves in benzene. Benzene is a nonpolar solvent with a molecular mass of 78 amu. These observations are inconsistent with the explanation(s) put forth by:
Your Answer is
Correct Answer is A
Explanation
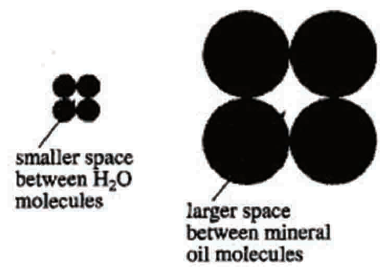
The two substances in the question stem are nonpolar, so they are compatible with Students 2 & 3;
According to the question stem, the 9,10- Diphenylanthracene can dissolve in benzene with a smaller molecular weight, which contradicts what Student 1 said