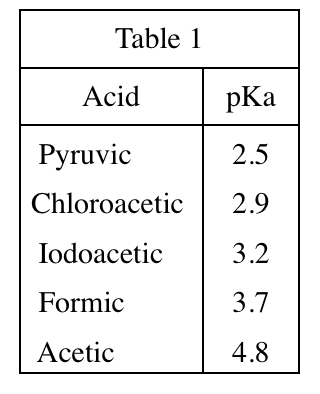
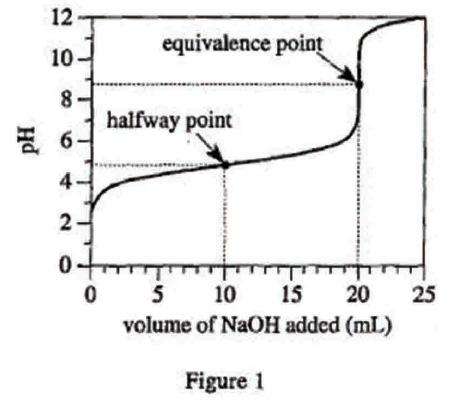
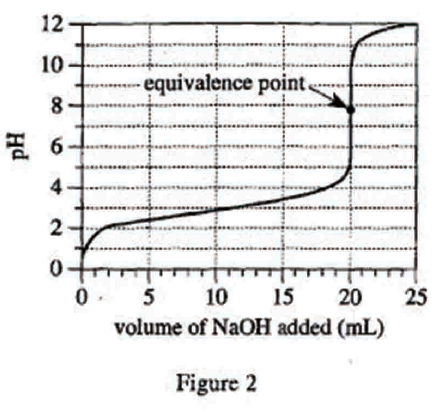
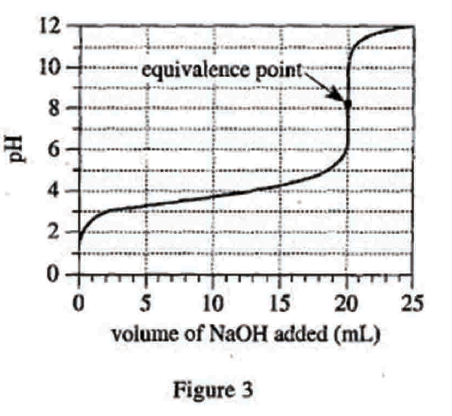
28. Suppose Experiment 3 is repeated, but the concentration of the NaOH solution is 0.200 mole/L. Will the volume of NaOH added at the halfway point and at the equivalence point be less than, greater than, or equal to the corresponding results shown in Figure 3 ?
Your Answer is
Correct Answer is F
Explanation
Looking at the first sentence of the text part of Experiment 1, we can see that the concentration of the NaOH solution used in the original experiment is 0.1mole/L, but the concentration given in the question stem is 0.2mole/L, which is higher. Therefore, to titrate the same acid with a higher concentration of base, only a smaller amount is needed to completely neutralize and reach the equivalence point. And the halfway point is the general equivalence point, so it only needs less amount