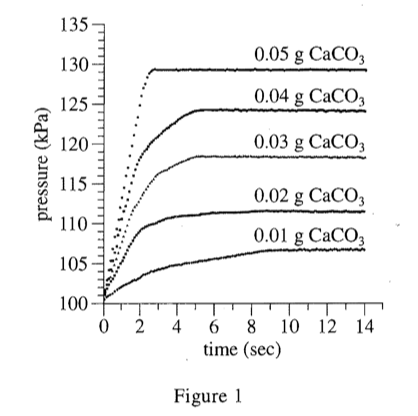
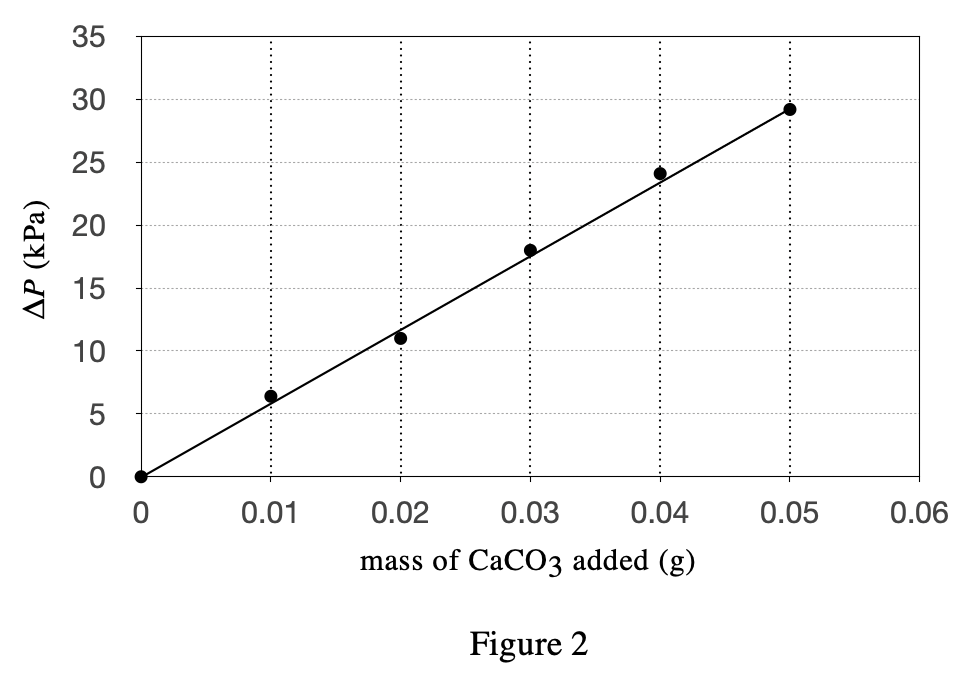
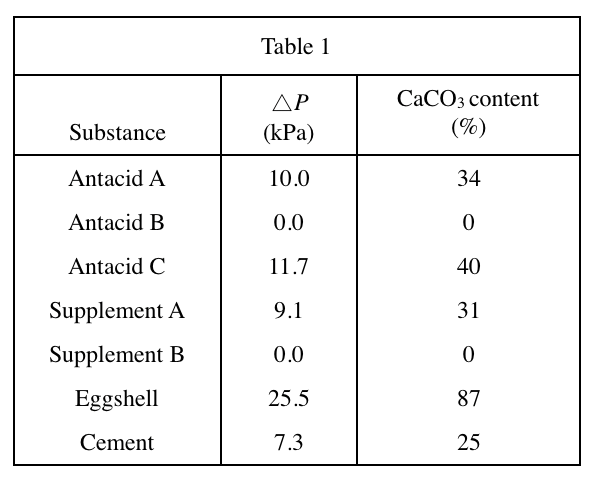
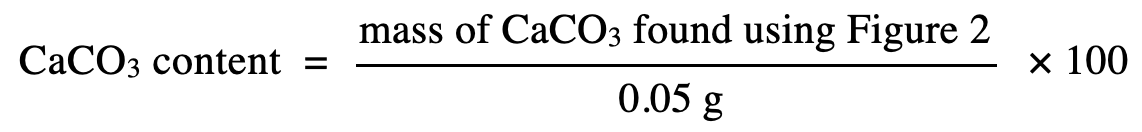40. Suppose 1 g of Supplement B were mixed with 1 g of eggshell to form homogeneous Powder X, and then 1 g of Antacid C and 1 g of cement were mixed to form homogeneous Powder Y. Based on Table 1, if Powders X and Y were each tested as in Experiment 2, which powder would have a higher △P ?
Your Answer is
Correct Answer is F
Explanation
The CaCO3 content of supplement B in Table 1 is 0%, the CaCO3 content of eggshell is 87%, and the CaCO3 of powder X is mixed content=(87%+0%)/2=43.5%;
Antacid C's CaCO3 content is 40%, cement's CaCO3 content is 25%, mixed into powder Y's CaCO3 content=(40%+25%)/2=32.5%;
So powder X's CaCO3 content is bigger, so △P should be bigger