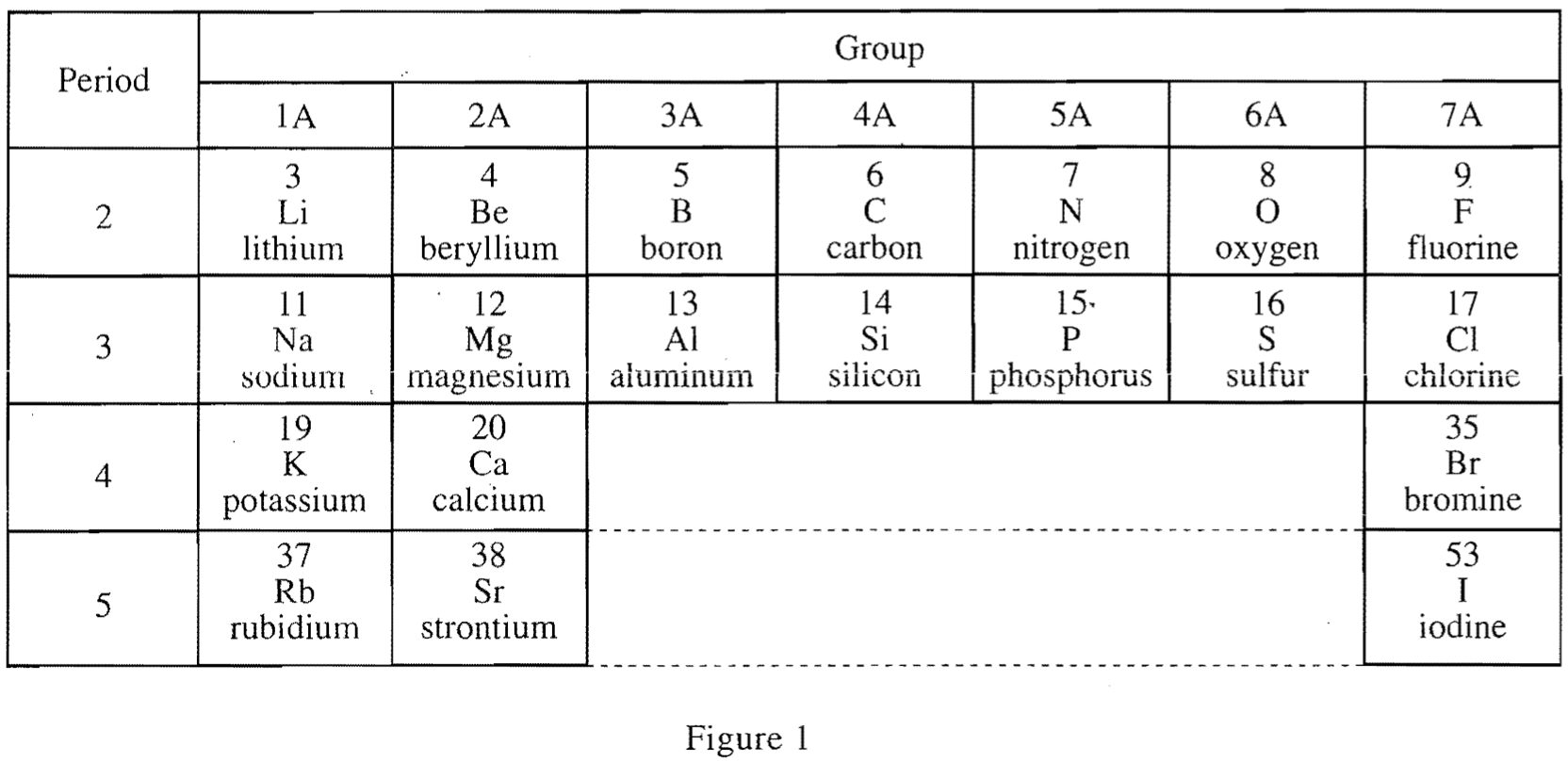
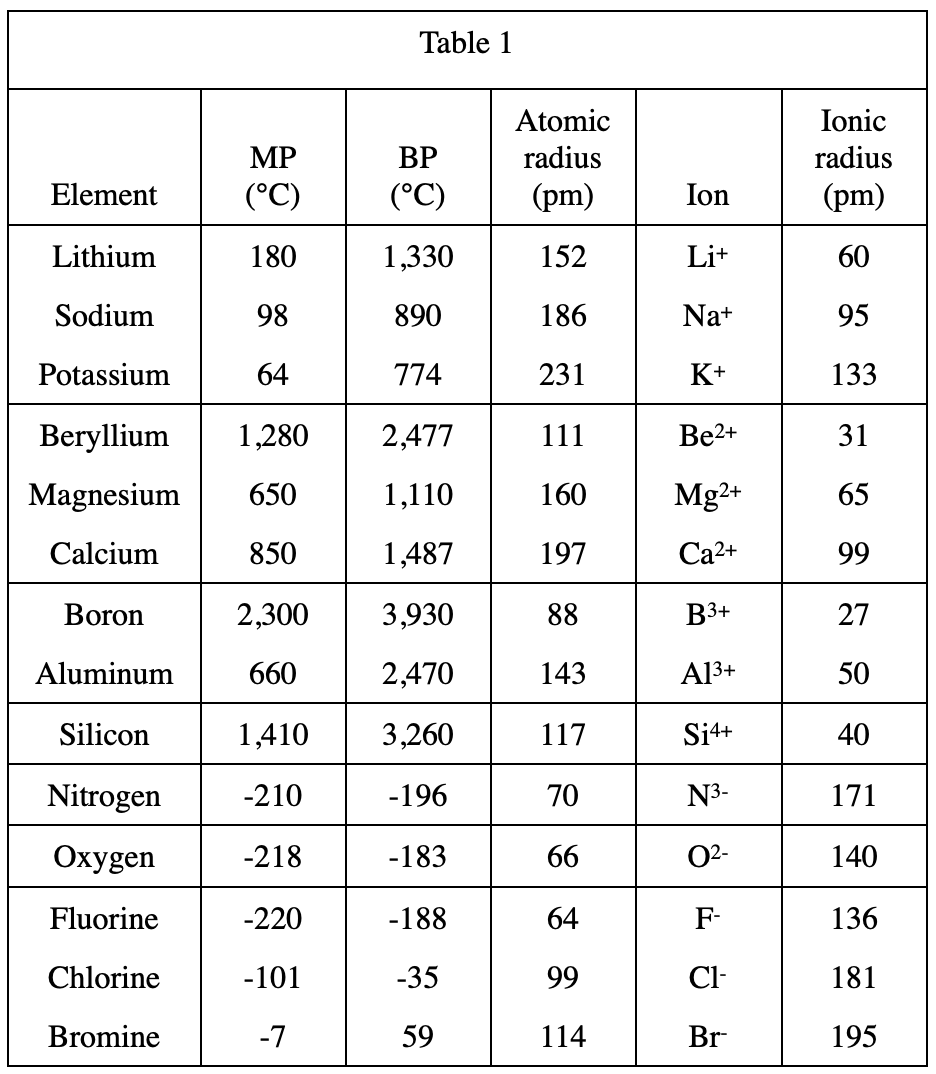
24. Which of the following statements comparing the ionic radius to the atomic radius for a given element is best supported by Table 1 ? Compared to the size of an atom, the size of the ion is:
Your Answer is
Correct Answer is J
Explanation
Comparing the atomic radium and ionic radius of each element in table 1, it can be seen that when the ion is positively charged, the ionic radius is smaller than the atomic radius;
When the ion is negatively charged, the ionic radius Both are larger than the atomic radius