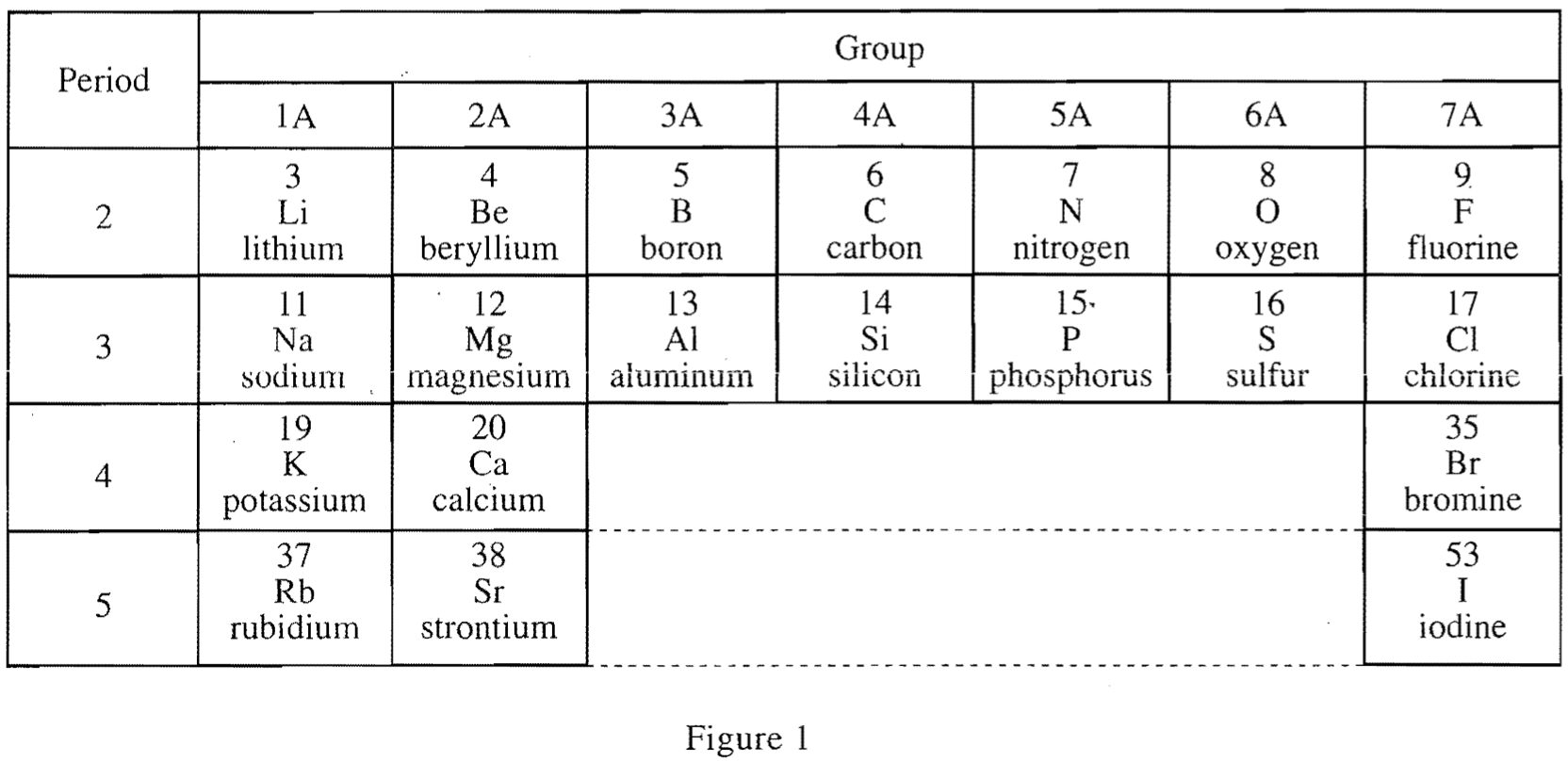
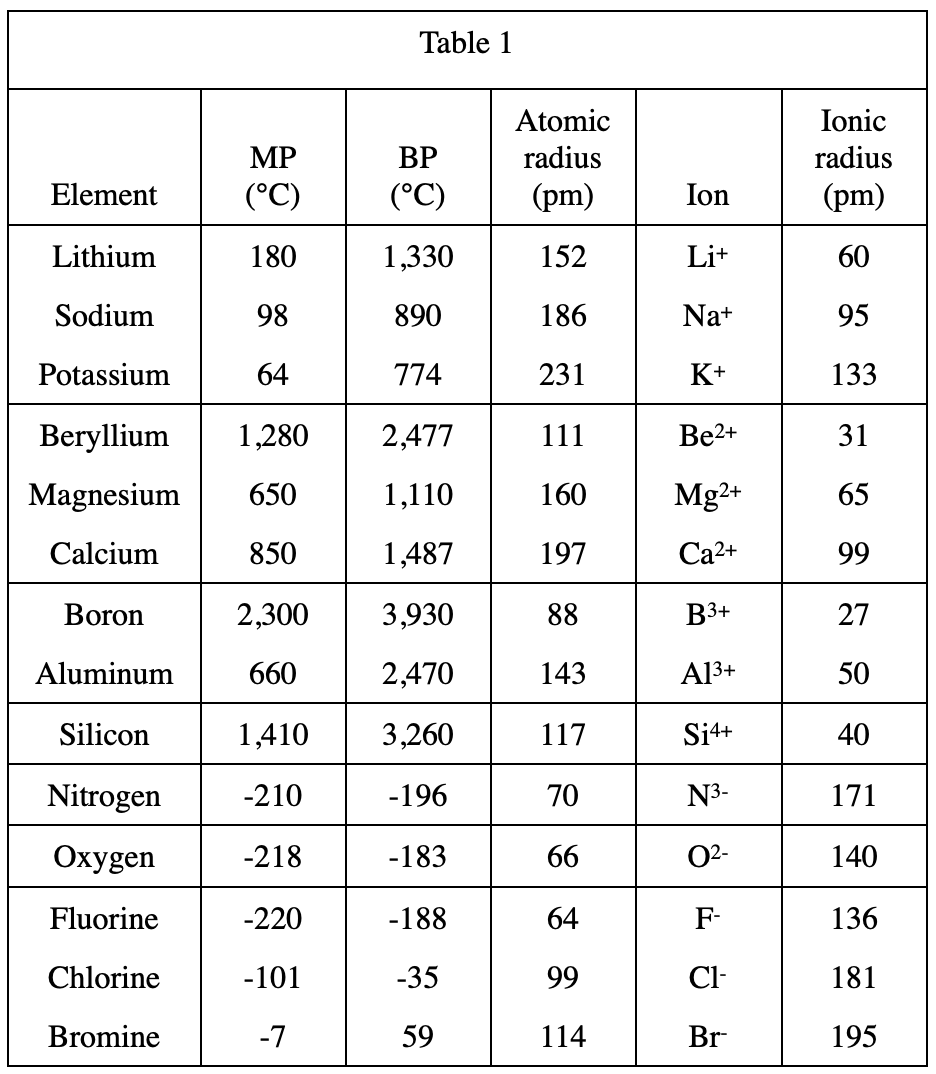
23. According to Figure 1 and Table 1, for the Period 3 elements in Groups 1A-4A and the ions listed for those elements, as atomic number increases, ionic radius:
Your Answer is
Correct Answer is C
Explanation
According to the period 3 and Groups 1A-4A mentioned in the question stem, the corresponding elements should be Na, Mg, Al, Si, and their Ionic radius decreases as the atomic number increases