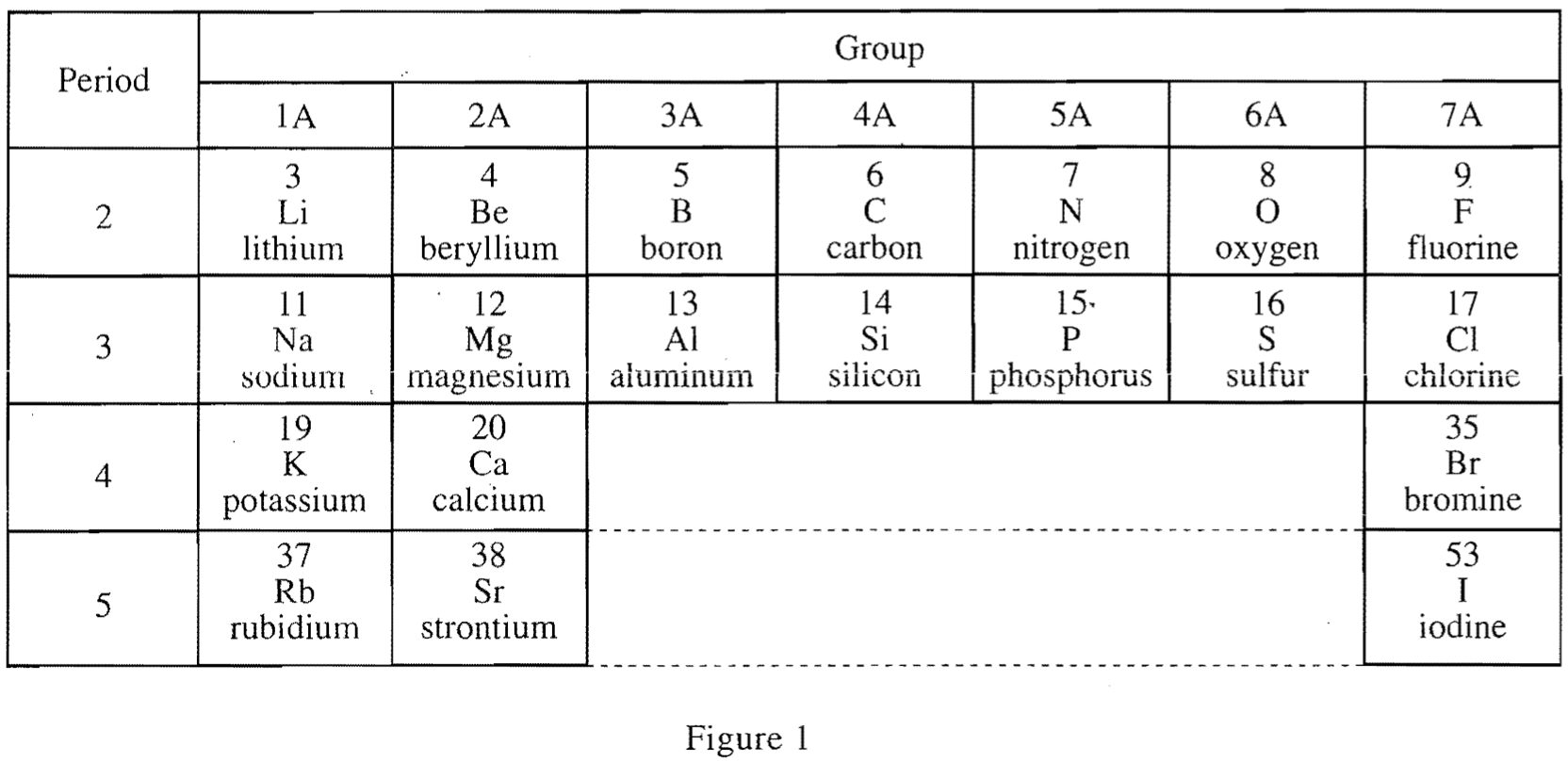
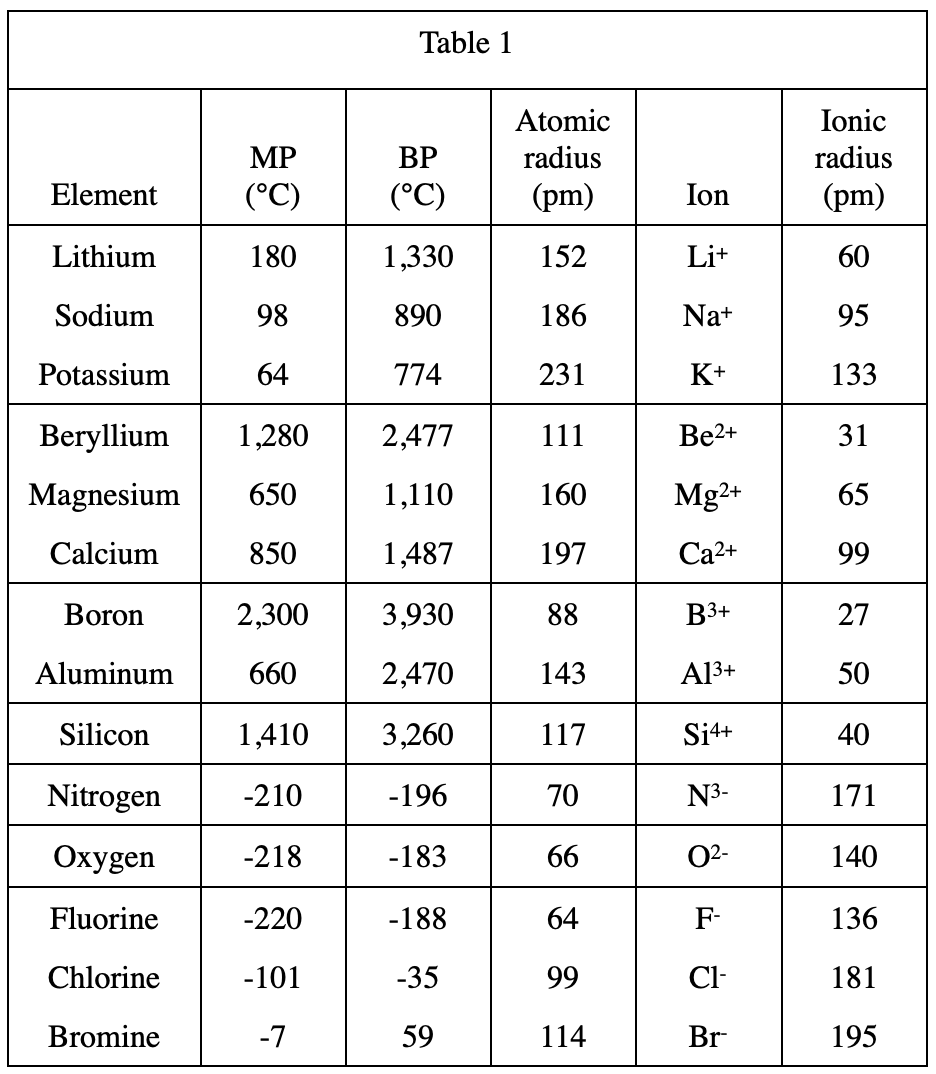
21. Barium (Ba), like Sr, is a Group 2A element. The atomic number of Ba is 56. Based on Figure 1 and Table 1, which of the following is the most plausible set of values for the atomic radius of Sr and of Ba ?
Your Answer is
Correct Answer is C
Explanation
It can be seen from table 1 that the greater the atomic number of an element in a specific Group, the greater the atomic radius;
So it can be predicted that the atomic radius of Sr is greater than that of Group 2A Ca's 197, and Ba's atomic radius should be larger, so option C is in line