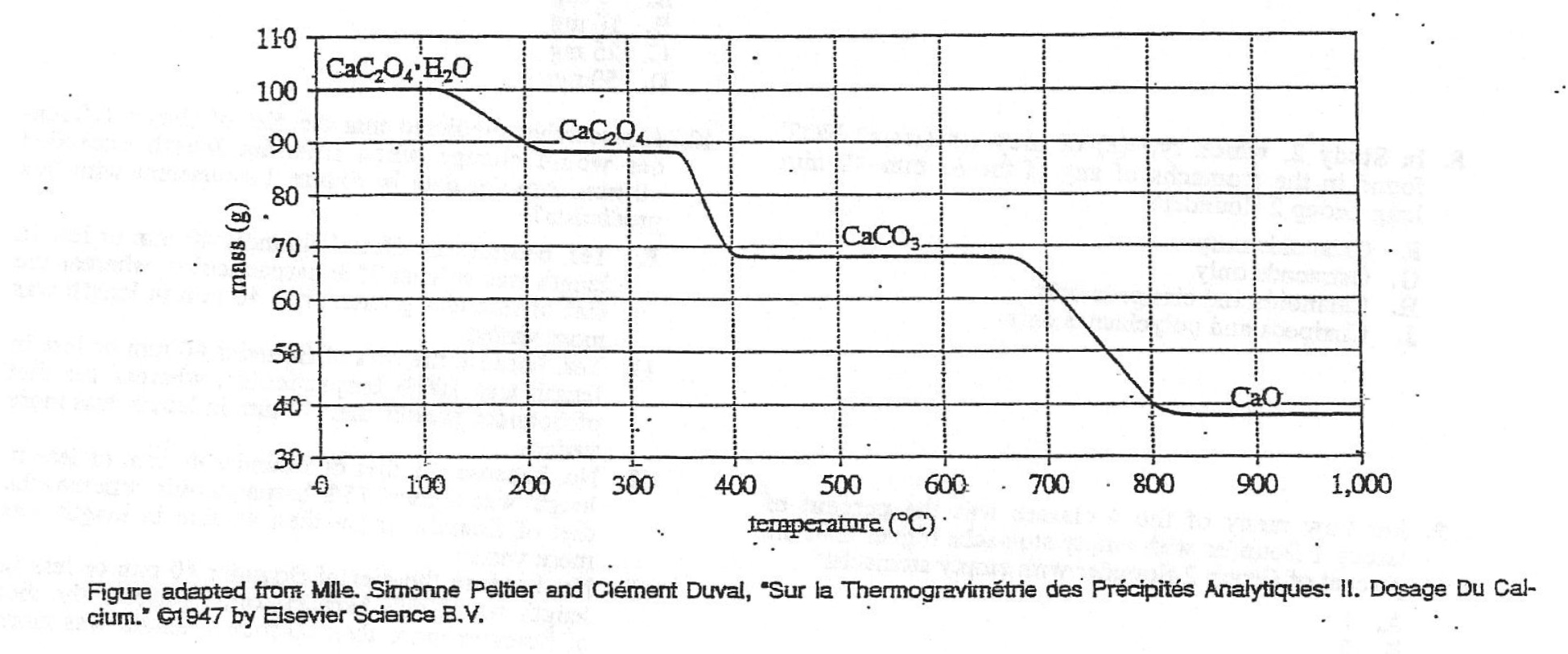
16. When the TGA began, approximately what percent of the mass of the sample was made up of H2O ?
Your Answer is
Correct Answer is F
Explanation
Method 1: A smart but common method for scumbags, reading pictures. The initial CaC2O4•H2O is 100 g, after decomposing and losing water, CaC2O< sub>4, the mass becomes about 88 g, and the mass of lost H2O is about 100-88=12 g, so the proportion of water is about 12/100
Method 2: Calculating the molecular mass in a stupid but learned way. The molecular mass of CaC2O4•H2O is 146, and the molecular mass of H2O is 18 , 18/146≈12%
 CaC2O4(s) + H2O(gas, g)
CaC2O4(s) + H2O(gas, g)