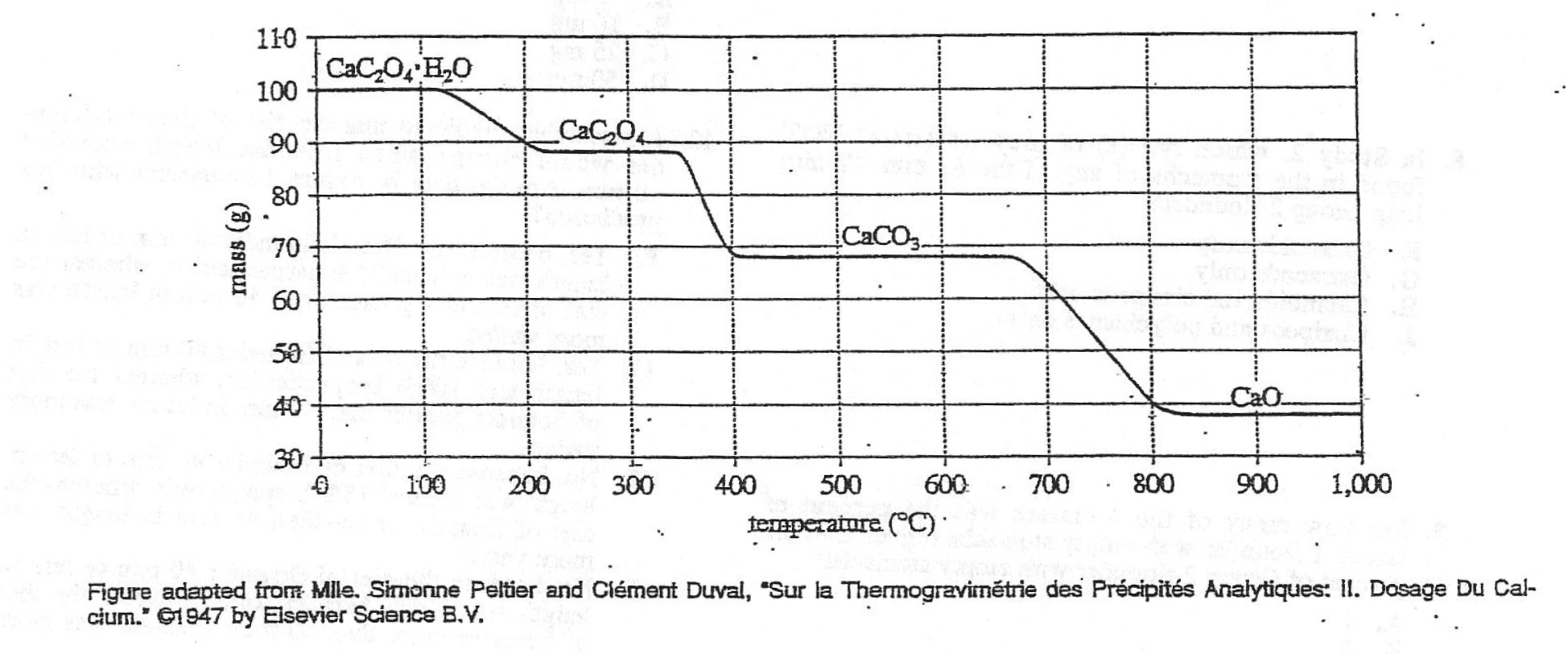
13. If the sample of CaC2O4·H2O had been 50 g, the mass of the sample at 900°C would have been approximately:
Your Answer is
Correct Answer is A
Explanation
Corresponding to the above figure, when CaC2O4•H2O is 100 g, and the abscissa temperature is 900, the reaction product The mass of CaO is about 38 g;
So when we have 50 g CaC2O4•H2O , the mass of CaO should be half of that on the picture, that is, 38/2=19 g, which is the closest to option A 20 g
 CaC2O4(s) + H2O(gas, g)
CaC2O4(s) + H2O(gas, g)