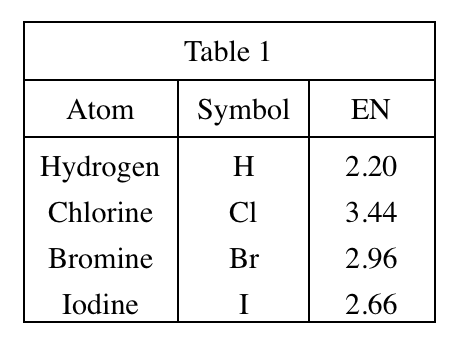
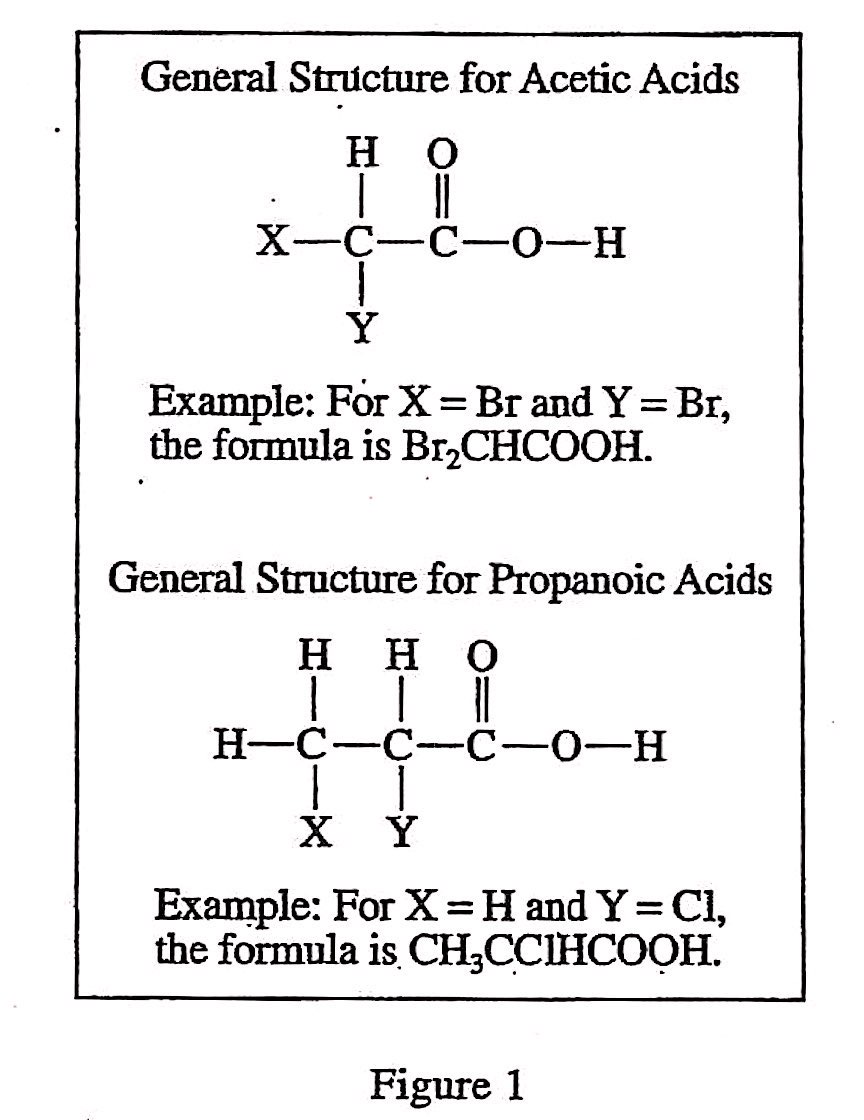
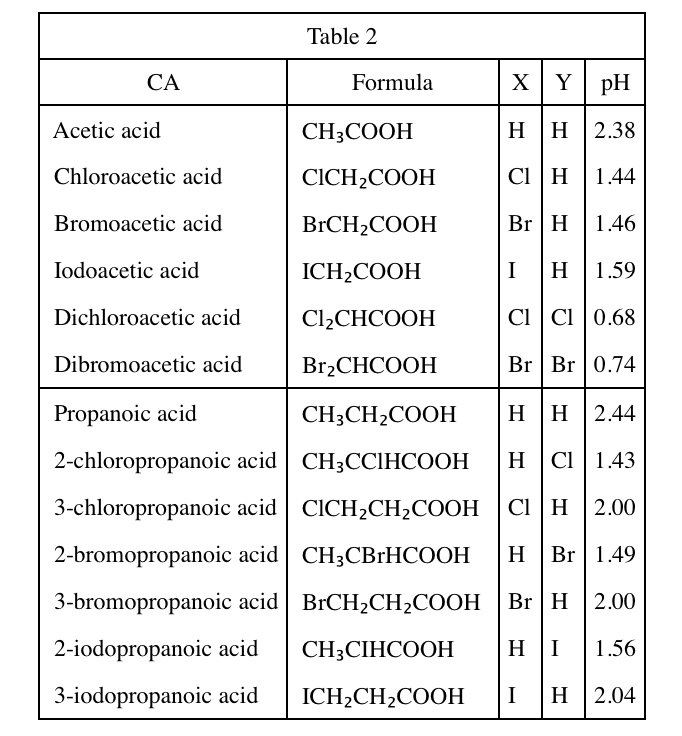
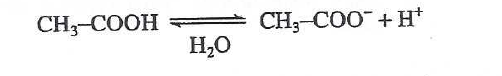40. Fluorine (F) is the most electronegative of all elements. Based on Tables 1 and 2, the pH of a 1.00 M aqueous solution of fluoroacetic acid at 25°C would most likely be:
Your Answer is
Correct Answer is F
Explanation
Comparing table 1 & table 2, it can be seen that in the group of actic acid, the stronger the electronegtivity of the atoms contained, the stronger the corresponding acidity.
According to the question, Fluorine has the strongest electronegtivity, so its acidity should be greater than that of cholroacetic acid, that is, the pH is less than 1.44